April 11, 2023
Click here to read “AI and Glaucoma Part I” and here to read “AI and Glaucoma Part III.”
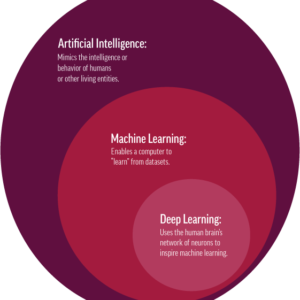
Artificial Intelligence (AI) can use fundus photographs to detect ophthalmic disease. This is according to a Gulshan et al. paper describing the validation and development of a deep learning algorithm for the detection of diabetic retinopathy using retinal photographs.1 The algorithm had a 97.5% sensitivity with a specificity at 93.4% to detect referrable diabetic retinopathy (moderate or worse). This early study showed how retinal photographs may be used to screen for diabetic retinopathy, performing as well as an excellent clinician.
FDA-Approved AI Can Detect Diabetic Retinopathy . . . Is Glaucoma Next?
From this work and others, two devices (the IDx-DR AI system from Digital Diagnostics and the EyeArt AI system from Eyenuk, Inc.) became FDA approved and commercially available for the detection of more than mild diabetic retinopathy in adults.2 A recent paper confirmed how an AI system, in this case the EyeArt AI system, performed at least as well as clinicians to detect more than mild diabetic retinopathy.3 Recently, Eyenuk received European Union MDR certification for autonomous detection of diabetic retinopathy, age-related macular degeneration, and glaucoma using the EyeArt AI system. The FDA has not yet approved any AI system for the detection of age-related macular degeneration or glaucoma, but this may be forthcoming.
A paper published in 2018 by Poplin et al. from the Google Research Group showed further possibilities for deep learning.4 In this paper, the age, gender, cigarette smoking status, HbA1c (diabetes) status, body mass index (BMI), and blood pressure measurements were predicted after analysis of retinal photographs. It is as if the study’s authors had listened to the American Academy of Optometry lecture by Dr. Jost Jonas 20 years ago and automated the process to draw information from a photograph. I am amazed that the algorithm was able to predict one’s gender based on a photograph.
Exploring the Possibility of AI/Deep Learning to Detect Glaucoma
These studies laid the groundwork for AI/deep learning, with papers exploring glaucoma diagnosis. A 2018 paper by Li et al. examined the performance of a deep learning algorithm to detect referrable glaucomatous neuropathy (cup/disc ratio of 0.7 or greater or other signs of glaucoma) based upon retinal photograph assessment.5 The sensitivity was 95.6% with a specificity of 92.0% with high myopia, diabetic retinopathy, and macular degeneration being reasons for falsely reporting glaucoma. Medeiros et al. used a different approach to assess glaucomatous damage seen in retinal photographs, training a deep learning algorithm based on OCT data to predict RNFL thickness.6 In other words, using only a retinal photograph, the algorithm was capable of predicting the RNFL without an OCT assessment.
Thompson showed that a deep learning algorithm can be trained using OCT minimum rim width to predict neuroretinal rim damage based upon retinal photographs.7 Using a deep learning model, Dr. Medeiros and colleagues were able to detect progressive RNFL change seen on optic nerve photographs and OCT images.8 Mariottoni and colleagues showed how a deep learning approach may predict visual field damage based on OCT RNFL thickness measurements.9 Hashimoto et al. used macula OCT and HFA 24-2 visual fields to construct 10-2 visual field maps, with the intent of reducing the need to perform additional field testing.10 These papers reveal possible uses of AI algorithms in confirming and measuring loss using fewer diagnostic tests.
Zhongwen et al. used widefield fundus imaging with a deep learning algorithm to detect glaucomatous optic neuropathy with sensitivity of 98.2% and a specificity of 94.3% with high myopia being the most common reason for false positive and negative results.11 Bowd et al. used OCTA images with deep learning to classify eyes as being healthy or glaucomatous.12 These papers reveal the versatility of deep learning programs to work with different diagnostic tools to assess for glaucomatous damage.
Machine Learning Can Predict Which Glaucoma Patients Will Progress
Regarding anterior chamber angle assessment, several studies have examined the use of anterior segment OCT images with AI to detect angle closure.13,14 Also, machine learning has been used to develop predictive modeling indicating which glaucoma patients may progress and do best with surgical intervention.15 This study used data from the electronic health record (EHR), including blood pressure measurements as well as systemic medications and showed an association between individuals who may require surgery, and which do not. The limitation for most studies using deep learning is the need for high quality images that are free of artifacts.
Based on these studies, it becomes clear that artificial intelligence and deep learning will become an important part of diagnostic testing.
References
1 Gulshan V, Coran M, Stumpe MC, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. 2016 JAMA; 316 (22):2402-24210.
2 Benjamens S, Dhunnoo P, Mesko B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digital Medicine 2020; (3):118.
3 Lim JI, Regillo CD, Sadda SVR, et al. Artificial intelligence detection of diabetic retinopathy. Subgroup comparison of the EyeArt system with ophthalmologists’ dilated examinations. Ophthalmology Science 2023; 3 (1).
4 Poplin R, Varadarajan AV, Blum K, et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nature Biomedical Engineering 2018; 2(3):158-164.
5 Li Z, He Y, Keel S, et al. Efficacy of a deep learning system for detecting glaucomatous optic neuropathy based on color fundus photographs. Ophthalmology 2018; 125(8): 1199-1266.
6 Medeiros FA, Jammal AA, Thompson AC. From machine to machine. An OCT-trained deep learning algorithm for objective quantification of glaucomatous damage in fundus photographs. Ophthalmology 2019l 126:513-521.
7 Thompson AC, Jammal AA, Medeiros FA. A deep learning algorithm to quantify neuroretinal rim loss from optic disc photogaphs. Am J Ophthalmol 2019; 201:9-18.
8 Medeiros FA, Jammal AA, Mariottoni EB. Detection of progressive glaucomatous optic nerve photographs with deep learning. Ophthalmology 2021; 128:383-392.
9 Mariottoni EB, Datat S, Dov D, et al. Artificial intelligence mapping of structure to function in glaucoma. Transl Vis Sci Technol 2020; 9(2): 19-31.
10 Hashimoto Y, Kiwaki T, Sugiura H, et al. Predicting 10-2 visual field from optic coherence tomography in glaucoma using deep learning corrected with 24-2/30-2 visual field. Trans Vis Sci Technol 2021; 10 (3):28.
11 Zhongwen L, Guo C, Lin D, et al. Deep learning for automated glaucomatous optic neuropathy detection from ultra-wide fundus images. Brit J Ophthalmol 2021; 105:1548-1554.
12 Bowd C, Belghith A, Zangwill LM, et al. Deep learning image analysis of optical coherence tomography angiography measured vessel density improves classification of healthy and glaucoma eyes. Am J Ophthalmol 2022;236:298-308.
13 Porporato N, Tun TA, Maskaran M, et al. Towards automated gonioscopy: a deep learning algorithm for 360 angle assessment by swept-source optical coherence tomography. Brit J Ophthalmol 2021; 106(10): 1387-1392.
14 Randhawa J, Chiang M, Porporato N, et al. Generalisability and performance of an OCT-based deep learning classifer for community-based and hospital-based detection of gonioscopic angle closure. Br J Ophthalmol 2021; online ahead of print.
15 Baxter SL, Marks C, Kuo T-T, et al. Machine learning-based predictive modeling of surgical intervention in glaucoma using systemic data from electronic health records. Am J Ophthmol 2019; 208:30-40.