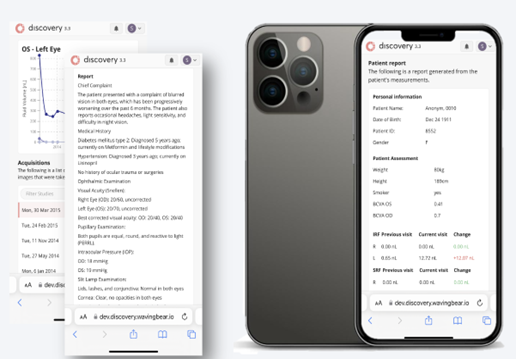
RetinAI Discovery Platform and AI Tools Aim to Improve Ophthalmic Care
February 26, 2024 BERN, Switzerland, and BOSTON — Ikerian AG and RetinAI Inc. US, a developer of software solutions for image processing and artificial intelligence (AI) in ophthalmology, has launched RetinAI Discovery for Clinics, with