July 18, 2023
NASHVILLE, Tenn. — Harrow Health, Inc. has announced agreements with affiliates of Santen Pharmaceutical Co., Ltd., under which Harrow will acquire certain U.S. and Canadian commercial rights for the following branded products from Santen:
U.S. Products:
- FLAREX (fluorometholone acetate ophthalmic suspension) 0.1%, a corticosteroid indicated for use in the treatment of steroid-responsive inflammatory conditions of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the eye.
- NATACYN (natamycin ophthalmic suspension) 5%, a sterile antifungal indicated for the treatment of fungal blepharitis, conjunctivitis, and keratitis caused by susceptible organisms, including Fusarium solani keratitis.
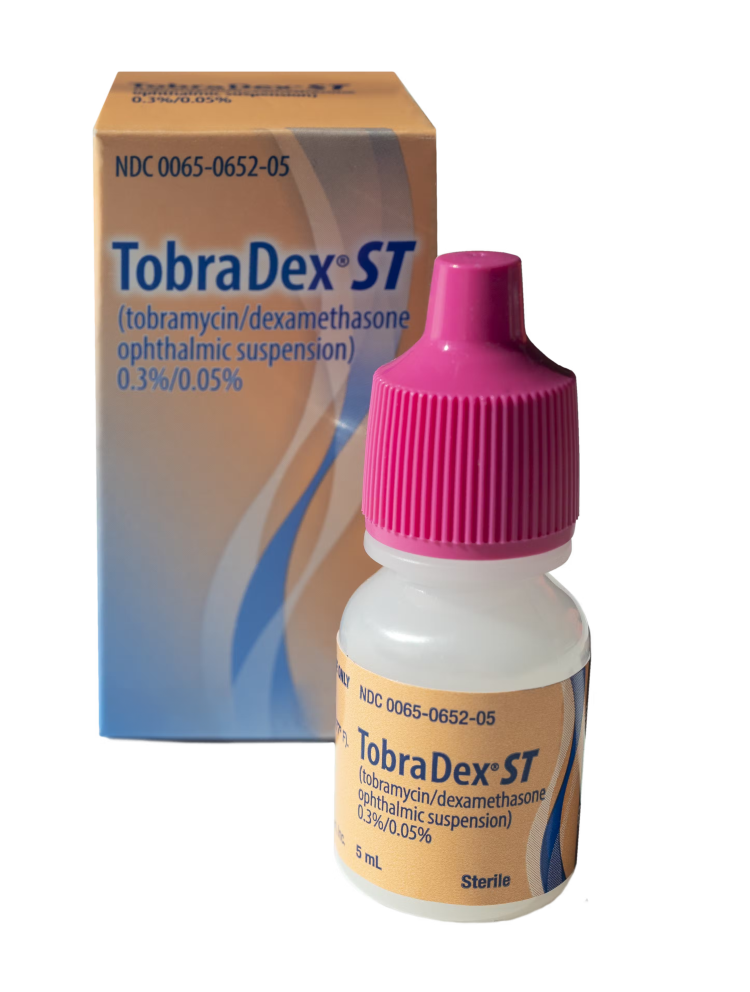
- TOBRADEX ST (tobramycin and dexamethasone ophthalmic suspension) 0.3%/0.05%, an antibiotic and corticosteroid combination for steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where superficial bacterial ocular infection or a risk of bacterial ocular infection exists.
- VERKAZIA (cyclosporine ophthalmic emulsion) 0.1%, a calcineurin inhibitor immunosuppressant indicated for the treatment of vernal keratoconjunctivitis (VKC) in children and adults and holds orphan-drug exclusivity.
- ZERVIATE (cetirizine ophthalmic solution) 0.24%, a histamine-1 (H1) receptor antagonist indicated for treatment of ocular itching associated with allergic conjunctivitis.
- FRESHKOTE, used as a lubricant to reduce further irritation or to relieve dryness of the eye.
Canadian Products:
- VERKAZIA (cyclosporine ophthalmic emulsion) 0.1%, a calcineurin inhibitor immunosuppressant indicated for the treatment of vernal keratoconjunctivitis (VKC) in children from four years of age through adolescence.
- Cationorm PLUS, a preservative-free emulsion for the treatment of dry eye symptoms and for the treatment of signs and symptoms of ocular allergy.
“This acquisition furthers Harrow’s goal of becoming a leader in the top tier of U.S. ophthalmic pharmaceutical companies, makes Harrow’s branded portfolio one of the most comprehensive in the U.S. market, and is expected to be immediately financially accretive upon the transfer of the product marketing authorizations,” said Mark L. Baum, Chairman and Chief Executive Officer of Harrow. “We are excited to add several high utility and trusted products that serve the ophthalmic surgical market, a market in which we already have a strong presence, and significantly expand the breadth of our portfolio, which will now include the only FDA‑approved ophthalmic antifungal; a patented and ‘orphan-designated’ product for the nearly 50,000 Americans suffering from the rare disease vernal keratoconjunctivitis (or VKC); a patented prescription drug to treat ocular itching associated with allergies; and two patented non-prescription brands serving patients managing dry eye symptoms.”