April 11, 2023
After a relatively quiet pandemic-era paucity in new ophthalmic FDA approvals, 2023 may be the year that the pipeline first trickles then flows into the clinic. Our industry registered a near strike out in 2022, with just a single ophthalmic agent registering a 2022 FDA approval (Omlonti omidenepag isopropyl from Santen Pharmaceutical Co., Ltd.*). This glaucoma product is a selective prostaglandin EP2 receptor agonist rather than working on the traditional FP type receptors with your traditional prostaglandin analogues.
A year earlier in 2021, only a handful were FDA approved, and few were truly new compounds or technology related to the delivery of medications. While I would never denigrate reformulations of existing drugs, our patients want and deserve more than the victory of taking a drug from 0.2% to 0.1% or what was previously twice daily becomes once daily.
I like to think about what is coming out to the market relative to my patient care needs. For example, when I see a patient with a certain condition, will the new upcoming FDA approval help my patients and how? You should be thinking the same way too. I make lists of patients to contact when a certain drug gets approved, mainly because I think the profile of the potential new drug and their associated data could help these patients.
What is the Process for FDA Approval?
When a drug is submitted to the FDA for approval, it is required to submit to nearly identical phase 3 clinical trials, demonstrating the same efficacy in achieving primary endpoints in the trial. Once these are completed and reviewed by the company, a face-to-face meeting between the sponsoring drug company and the FDA usually occurs. If there are no items requiring further study, then the sponsor can submit its final package to the FDA for approval.
Upon FDA review, a positive sign for approval is when a PDUFA date is announced, which means the drug is accepted for review and may get final approval on or prior to this date (generally 10 months or six months for a granted priority review). It’s important to note that the sponsor also pays a significant fee, upwards of $3.5 million to the FDA in concordance with the regulations associated with congressional authorization of the PDUFA Act. It is reauthorized every five years, and President Biden just reauthorized the act through FY2027.
What FDA Approvals Can We Expect in 2023?
Here are my undercover takes on some of the hottest potential approvals in 2023 based on PDUFA activity:
Intravitreal pegcetacoplan from Apellis Pharmaceuticals, Inc. After decades of intravitreal injectable technology for exudative macular degeneration and other neovascular disorders, we finally have some molecules on the way for geographic atrophy (GA) in macular degeneration. While this formulation was originally issued a November 2022 PDUFA date, the FDA approved the drug in February 2023 and issued them the name SYFOVRE. Their two phase 3 studies (DERBY and OAKS) were augmented with 24-month extension data submitted as an NDA amendment. The agent works by targeting the C3 mechanism, which is upregulated in GA to cause excessive activation of the complement cascade modulated through the immune system. C3 seems to regulate lesion growth rate. With the average time of 2.5 years for the average GA lesion to grow and encroach on the fovea, this C3 targeted system is our first foray into GA treatments and surely one of the most important events in the retinal specialty space. It is one of the most important events in optometry given the fact that we manage the vast majority of these patients due to a historical lack of any FDA-approved therapies for GA.
NOV03 perfluorohexyloctane from Bausch + Lomb, Inc. This PDUFA action date is set for June 28, 2023. The journey of managing meibomian gland dysfunction (MGD) is seemingly just getting started. During my training in the mid ‘90s, MGD just didn’t exist in my textbooks or anterior segment disease courses. You learned there were meibomian glands in the eyelids, but their importance in ocular surface disease was never primary. Fast forward and now we have automated and instrument-assisted heating and evacuation of meibomian glands and a topical medication specifically for MGD, finally! Bausch + Lomb in-licensed NOV03 from Novaliq GmbH.
Pharmacologically speaking, NOV03 is a very different type of agent. There isn’t a vehicle or even a concentration for this drug; it is 100% perfluorohexyloctane. It is water, steroid, and preservative free. Bausch + Lomb is seeking an indication for excess tear evaporation as well as the signs and symptoms of dry eye disease associated with MGD. Both phase 3 clinical trials (GOBI and MOJAVE) met all their primary and secondary symptom and sign endpoints as early as two weeks and through 57 days. I look forward to using this in practice, especially considering the quick onset of efficacy in the clinical trials.
The Biggest Unmet Need in Clinical Eye Care — Blepharitis
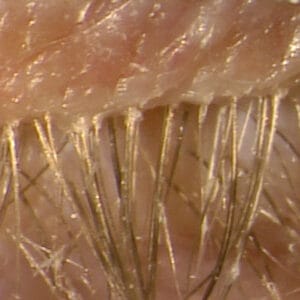
TP03 lotilaner ophthalmic solution 0.25% from Tarsus Pharmaceuticals. This PDUFA date is set for September 2023. What is the biggest unmet need in all clinical eye care? This undercover clinician says, without question, it is blepharitis. Clinical trial data from the recently published Atlas and Titan studies highlight the omnipresence of blepharitis. Nearly two-thirds of all patients presenting to an eye care practice had slit-lamp-proven blepharitis. Patients with glaucoma have it, dry eye patients have it, and contact lens dropouts have it. Demodex blepharitis causes plenty of problems, most of which are indistinguishable from other characteristics of ocular surface diseases. “Cosmetic, emotional, physical, and hard to put your finger on” is how I describe blepharitis.
Plain and simple, blepharitis is caused by demodex infestation. I know that can and will be debated, but we can address that after we have an opportunity to work with what could be a game-changing medication in TP03. Tarsus completed two phase 3 clinical trials (SATURN-1 and SATURN-2) showing safety and efficacy outcomes for over 800 patients. TP03 met the primary endpoint as well as all secondary endpoints with statistical significance. The primary endpoint of complete collarette cure (less than or equal to two collarettes per lid) was over 56% in SATURN-2. Statistically significant improvements in secondary measures included lid erythema scores, clinically meaningful collarette cure, and complete mite eradication microscopically. In addition, over 90% of patients in both phase 3 trials had at least one grade improvement in collarette scores after treatment.
2023 Will See Weapons Against Three Brutal Conditions
What if I told you that in 2023, we would likely have weapons against three brutal, life-altering conditions? We have a shot on goal for dry macular degeneration, MGD, and dry eye, as well as the ubiquitous and irritating demodex blepharitis. Two of the three agents can possibly show anterior segment symptom improvement within two weeks of starting therapy!
For dry age-related macular degeneration, I’m already talking to patients daily about new options coming their way instead of just OTC vitamins. While that may have seemed unbelievable in the past, they will be here before you know it.
* The launch of Omlonti is on hold due to the restructuring of Santen Pharmaceutical Co., Ltd.
Disclosure: Ian Benjamin Gaddie, OD, FAAO, is the lead author on SATURN-2 publication.