May 25, 2023
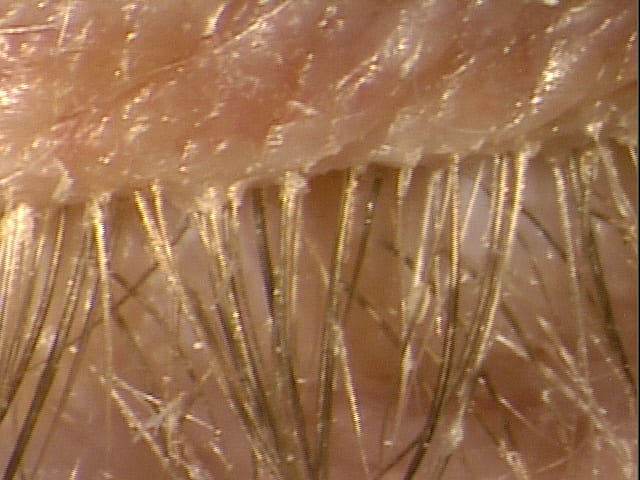
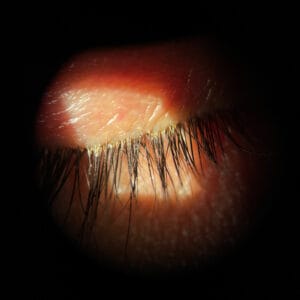
Since at least 1915, when Cornell researchers William A. Riley and O.A. Johannsen first described the anatomy and classification of demodex mites, doctors and patients alike have been waiting for a treatment for these pesky critters that cause so much trouble on the surface of the lids. Generations of eye care providers have heard the stories of these mythical bugs only to never really see or appreciate their presence despite seeing 40,000 or more patients during their careers. Often confused with other parasites that can reside in the lashes on an itinerate basis, the demodex species is so small that even under the highest of slit lamp magnifications it is a miracle to see one. In a recent observational study (Titan), the prevalence of collarettes, the pathognomonic sign of demodex blepharitis, among patients seeking eye care for any reason, was reported as 58%. This means that an estimated 25 million U.S. adults of the 45 million who visit eye care professionals annually have demodex blepharitis. The market is enormous, and it is, in my opinion, one of the largest unmet needs in all of eye care.
The decade of revolution in demodex diagnosis was 2010-2020. Inexpensive microscopes purchased on Amazon could be used toview extracted lashes with collarettes in the quest to see that live demodex doing the backstroke. You could hear gasps, cries, and screams in the hallways of clinics as it was revealed that someone has “the bugs.” Now, microscopic images of these mites can be seen on the screen at almost any CME event in the eye care space.
Finally, the Treatment for Demodex Blepharitis
 In 2020, Tarsus Pharmaceuticals began the commercialization of its TP-03 program with the isoxazoline class compound lotilaner. Originally developed for veterinarians for the treatment and prevention of fleas and ticks, lotilaner results in rapid paralysis and death of the mite. It is a well characterized anti-parasitic drug that works by selectively inhibiting the GABA-CL channels. It is highly lipophilic, which should allow for uptake in the sebum of the hair follicles. The promise of this drug opens the possibility that for the first time we may have an actual sustainable treatment and, in many cases, a collarette cure.
In 2020, Tarsus Pharmaceuticals began the commercialization of its TP-03 program with the isoxazoline class compound lotilaner. Originally developed for veterinarians for the treatment and prevention of fleas and ticks, lotilaner results in rapid paralysis and death of the mite. It is a well characterized anti-parasitic drug that works by selectively inhibiting the GABA-CL channels. It is highly lipophilic, which should allow for uptake in the sebum of the hair follicles. The promise of this drug opens the possibility that for the first time we may have an actual sustainable treatment and, in many cases, a collarette cure.
With the pivotal phase three clinical trials of this drug and an FDA PDUFA date in late August of 2023, the noise has been ramped up. As a result, and to be fair, there is a lot of disease state awareness that still needs to happen. I’m surprised when I peruse eye-specific social media how that few cases presented with ocular surface disease mention the presence of demodex or blepharitis for doctors seeking advice. Sometimes pictures are posted, and routinely I’m immediately drawn to the collarettes that are just so obvious. I’m hoping that with a reasonable treatment available we will finally see the impact of eradication of these pesky creatures.
Perhaps this is where the divergence of science, psychology, and plain fear occurs. Both fear and angst result amongst patients upon hearing their diagnosis, and providers often opt to use fear to ensure compliance. In my opinion, there is no question about the science of demodex blepharitis. Simply look at all the publications in the last three years on the topic.
Here are some examples:
Diagnosis and Management of Blepharitis
Smite the Mites! How to Treat Demodex Blepharitis
This is where psychology needs to be considered and appreciated. Believe it or not, patients can freak out when learning of their diagnosis. I’ve been there plenty of times already, and it should make you think about your approach. For some, I pull up images of demodex blepharitis, and then I take a photo of their lids to compare and discuss. Sometimes I pull up images of the mites themselves, but this depends on reading my patient and their personality. If we aren’t careful, we could have a zombie-like apocalypse of patients going neurotic about bugs on their bodies!
I think this will be the crux of mitigating what is truly the missing link in managing ocular surface disease (OSD). Think about all those patients of yours who have what we have historically considered to be dry eye or OSD symptoms. I have so many patients who faithfully take their cyclosporine/lifitegrast and/or steroids who still are not over the hump from a symptom standpoint. Perhaps they are better, but they are still suffering. If you are listening well, they are telling you what is going on. Of course, you must ask specific dry eye questions, and I highly suggest the SPEED questionnaire, which is free and easy for patients to answer. After the tech work up, they fill out the survey as I make my way into the room to see them. The worst symptom score is 28/28. My cutoff to ask and probe with more questions is an 8. Simply put, patients don’t know how to articulate OSD symptoms, which is a major contributor to why this overall OSD population continues to go undiagnosed.
Diagnosing Demodex Blepharitis
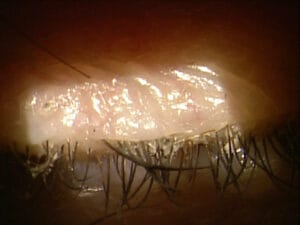 When clinically diagnosing demodex blepharitis, the good news is you don’t need a single piece of new equipment. You only need your basic slit lamp. I don’t care where in your slit lamp sequence you add this step, but the patient needs to just look down. For me, it’s after I do my normal sequence as a last step, simply asking the patient to “look down.” Almost all immediately oblige, and you can instantly see any evidence of a collarette. Sometimes I have to search a little harder for scant evidence of collarettes, but usually this step takes me seconds and never more than two to three seconds. Just a solitary collarette can confirm the presence of demodex without need for microscopic confirmation. That’s it! Sometimes there are aspects such as coexistent seborrheic blepharitis and rosacea. While related, the collarette is the main diagnostic marker needed to make the call. It causes eyelid margin inflammation, redness, and ocular irritation. It may lead to dry eye, red, itchy, or irritated eyelids, missing or misdirected eyelashes, inflammation of the conjunctiva and lid margin, and recurrent chalazia.
When clinically diagnosing demodex blepharitis, the good news is you don’t need a single piece of new equipment. You only need your basic slit lamp. I don’t care where in your slit lamp sequence you add this step, but the patient needs to just look down. For me, it’s after I do my normal sequence as a last step, simply asking the patient to “look down.” Almost all immediately oblige, and you can instantly see any evidence of a collarette. Sometimes I have to search a little harder for scant evidence of collarettes, but usually this step takes me seconds and never more than two to three seconds. Just a solitary collarette can confirm the presence of demodex without need for microscopic confirmation. That’s it! Sometimes there are aspects such as coexistent seborrheic blepharitis and rosacea. While related, the collarette is the main diagnostic marker needed to make the call. It causes eyelid margin inflammation, redness, and ocular irritation. It may lead to dry eye, red, itchy, or irritated eyelids, missing or misdirected eyelashes, inflammation of the conjunctiva and lid margin, and recurrent chalazia.
This is where you immediately see convergence of symptoms overlap between dry eye/OSD and demodex blepharitis. Common symptoms include tearing/watery eyes, eye and eyelid itching, red eyes and eyelids, crusty eyes, blurred vision, and foreign body sensation.
Ocular itching is a common complaint that most practitioners attribute to allergy. If you take time to ask your patient where the eye is itching, they will often reach up to demonstrate a horizontal itching motion over the eyelids. To me, that is a tell-tale demodex symptom. The other one to mind is eyelid redness. This is a much-underappreciated symptom. Think about aesthetics and eye redness. Lumify has dominated this market since launch, however I think a significant amount of eyelid redness comes from demodex blepharitis. In the pivotal trials for TP-03, this was one of the factors that improved vs. vehicle. This is one of the more subtle real-world symptoms I’ll be anxious to see how it is impacted with TP-03.
Impressive Data Result from Saturn-1 and Saturn-2 Clinical Trials
As is standard with New Drug Applications and phase three clinical trials, Tarsus sponsored two identical phase three clinical trials of TP-03 vs. Vehicle (Saturn-1 and Saturn-2). Both studies went out to day 43, and Saturn-2 had a one-year safety data follow-up as well. The studies included approximately 412 patients who had at least 10 collarettes per lid and at least mild eyelid hyperemia, each randomized to receive TP-03 or vehicle twice daily for six weeks. There was no treatment for blepharitis, including eyelid hygiene, for 14 days before and during the term of the study. The primary endpoint was a complete collarette cure, defined as 0 to 2 collarettes per lid at day 43. [I was the primary author on the submission of data for Saturn-2.]
The Saturn-1 data is very similar with identical p-values for both studies. For Saturn-2, this was achieved in 56% of TP-03 patients vs. 13% on vehicle (p<0.0001). In addition, 89% of TP-03 patients achieved a significant, clinically meaningful collarette cure defined by a collarette grade of 0 or 1 at day 43 vs. 33% of vehicle-treated patients (p<0.0001). Secondary endpoints in Saturn-2 included mite eradication (measured by microscopy and defined as zero mites per lash), and complete lid erythema cure and complete composite cure (achieving both collarette cure and erythema cure). For mite eradication, 52% of TP-03-treated patients achieved zero mites per lash at day 43. Complete lid erythema score was obtained in 31.1% of TP-03-treated patients compared to 9% of vehicle patients (p<0.0001). Complete composite cure was achieved in 19.2% of TP-03-treated patients compared to 4% on vehicle (P<0.0001). The safety profile demonstrated good tolerability with a safety profile similar to vehicle, and 91% in Saturn-2 reported that the drop comfort was neutral to very comfortable. There were no serious treatment-related adverse events. The only adverse events greater than a rate of 1% in the TP-03 group were instillation site pain/burning/stinging (7.9%, n=16) and dry eye (1.5%, n=3).
While these data look impressive, we don’t know how long or persistent this treatment will be effective in reducing the load of demodex and their symptoms. One important note on the trials: No symptoms were evaluated pre or post treatment, meaning we just saw the clinical improvement in collarettes, redness, and mite counts but no patient input on how their eyes look or feel. This goes back to that psychology mentioned earlier. Do you just tell the patient they have an infection and prescribe TP-03, or are we going to go into an entire bug discussion with everyone we treat? I think it will depend on the doctor’s ability to know their patients and how best to discuss the diagnosis and get it treated.
How Are We Going to Make Our Decision to Treat?
Some will treat everyone with a collarette (or try to). Others will not pull the trigger unless it is a Grade 3 or 4 at baseline. Others will rely on symptoms to initiate treatment with TP-03. I have so many symptomatic patients who are awaiting treatment right now that I won’t have to ponder my decisions starting out. As time goes on, I’m hopeful that my success with early symptomatic patients will help guide my treatment initiation decisions on those with less symptom burden. Are we going to treat asymptomatic Grade 1 patients?
Finally, consider how long the six-week treatment will be durable? Will it be six months, eight months, one year or more? I suspect that most patients will reach six months with treatment durability, and perhaps 50% or more will make it one year or more. I can live with knowing some patients may need a second dose of a six-week BID drop. At least it isn’t a chronic, everyday medication with compliance and adherence issues like other chronic dry eye medications. And it begins working fast, in about two weeks based on the Saturn-1 and 2 data. If you’re like me, this much needed treatment can’t get here soon enough. I’m itching for it.
References
Brandon D. Ayres, Eric Donnenfeld, Marjan Farid, Ian Benjamin Gaddie, et al. Clinical diagnosis and management of Demodex blepharitis: the Demodex Expert Panel on Treatment and Eyelid Health (DEP)
Farid M, Ayres BD, Donnenfeld E , Gaddie IB, et al. Delphi Panel Consensus Regarding Current Clinical Practice Management Options for Demodex blepharitis. Dove Press , 27 February 2023, Volume 2023:17: Pages 667—679
Rhee, Michelle K. M.D, et al. Demodex Blepharitis: A Comprehensive Review of the Disease, Current Management, and Emerging Therapies. Eye & Contact Lens: Science & Clinical Practice ():10.1097/ICL.0000000000001003, June 2, 2023. | DOI: 10.1097/ICL.000000000000100