January 10, 2024
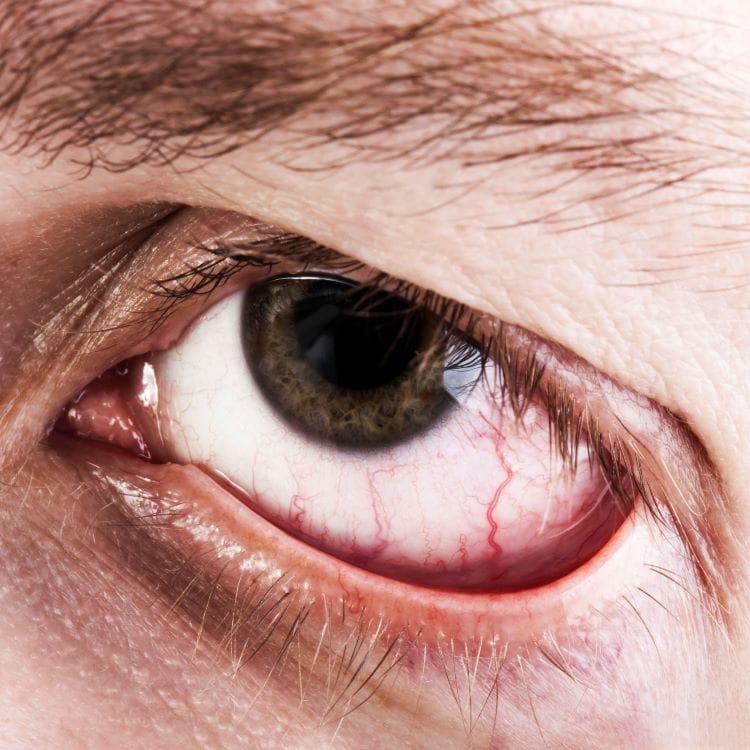
LONDON and NEW YORK — OKYO Pharma Limited reports positive safety and efficacy results in its Phase 2, randomized, double-masked, placebo-controlled trial evaluating the safety and efficacy of OK-101 ophthalmic solution in subjects with dry eye disease (DED). This first-in-human trial of OK-101 established a clear and informed path for further development in Phase 3 registration trials.
The double-masked, randomized, placebo-controlled Phase 2 trial was conducted at six sites in the U.S. and enrolled 240 subjects with DED dosed twice-daily (BID). Patients were randomly divided into three cohorts, with one of the cohorts dosed with 0.05% OK-101 (n=80), a second with 0.1% OK-101 (n=80), and the third cohort with vehicle (n=80). The duration of a patient’s treatment was 14 weeks, including a two-week run-in period on placebo, to exclude placebo responders from the study, followed by 12 weeks in the randomized portion of the study.
Highlights of OK-101 Phase 2 Trial
- OK-101 First-In-Human trial establishes a clear clinical path for further clinical development in a Phase 3 study design using FDA-recognized endpoints per the Dry Eye: Developing Drugs for Treatment Guidance for Industry.
- OK-101 demonstrated superiority when compared to placebo in the sign endpoint of total conjunctival staining as measured by the Ora Calibra Staining Scale as early as Day 29 (p = 0.034).
- OK-101 demonstrated superiority when compared to placebo across at least two symptoms of DED, including burning measured by the Ora Calibra Ocular Discomfort and 4-Symptom Questionnaire as well as burning/stinging measured by a visual analogue scale as early as day 15 (p = 0.04 and p=0.03, respectively). A statistically significant improvement in blurred vision was also achieved at day 29 (p = 0.01).
- Treatment emergent adverse events (TEAEs) were observed to be similar to the placebo-treated group. No severe drug-related ocular TEAEs were seen. Possible drug-related TEAEs were observed in one patient in the OK-101 0.05% treatment group and three patients in the placebo-treated group, again highlighting the favorable safety profile of OK-101.
- Additionally, fewer subjects in the OK-101-treated arm discontinued study medication (2.5%) compared to discontinuations in the placebo-treated patients (3.8%).
“It is remarkable that in this first in-human study of OK-101 ophthalmic solution, an analysis of all randomized subjects demonstrated a persistent, statistically significant improvement in multiple dry-eye-related symptoms as early as day 15, along with a sign, total conjunctival lissamine green staining, by day 29,” said Jay Pepose, MD, PhD, founder and medical director of Pepose Vision Institute and Professor of Clinical Ophthalmology at Washington University School of Medicine in St. Louis.
“Ameliorating this unique constellation of signs and symptoms may reflect the differentiated mechanism of action of OK-101. Also of note, drop comfort was excellent and the safety profile favorable. This is evidenced by fewer subjects discontinuing study medication in the OK-101 arm (2.5%) than the placebo arm (3.8%) due to treatment-emergent adverse events. This highly favorable tolerability profile is very significant because many patients commonly discontinue currently available dry eye medications due to unwanted side effects, such as blurred vision, conjunctival redness or altered taste sensation (dyseguesia).”
OKYO Pharma CEO Gary S. Jacob, PhD, stated in the news release, “We were pleased to identify both a statistically significant ‘sign’ endpoint – ‘total conjunctival staining,’ as well as two statistically significant ‘symptom’ endpoints – ‘stinging/burning’ and ‘blurred vision’ in our first trial in man. These results are extremely encouraging and will steer the selection of the future primary endpoints of subsequent Phase 3 trials of OK-101 to treat DED.”
Dr. Jacob continued, “Moreover, we were pleased to see these benefits showing up as early as 15 days after dosing, along with showing meaningful durability until the end of the trial. While we didn’t meet the original primary endpoints, the totality of the data support advancing forward with a new study design that leverages our learnings of the novel mechanism of action of OK-101. We plan to advance OK-101 into Phase 3 clinical trials, with the goal of developing a highly differentiated dry eye product to help patients underserved by current treatments. These results also further support the potential of OK-101 for the treatment for corneal neuropathic pain, which is our parallel development focus for OK-101 in 2024.”
Dr. Raj Patil, CSO of OKYO Pharma, said, “To our knowledge, there are no FDA-approved DED drugs that have been shown in clinical studies to improve conjunctival staining, a primary ‘sign’ endpoint for DED drug approval. OK-101 is a drug with multiple novel and differentiated mechanisms of action that include anti-inflammatory activity and restoring the mucin-secreting goblet cells in conjunctiva, as observed in animal models of DED.”
Dr. Patil added, “In addition, we see improvement in blurred vision, a primary ‘symptom’ endpoint for DED drug approval, in OK-101-related DED patients that is plausibly tied to enhancing the tear film stability and lubrication of the ocular surface by normalizing the mucin component in the tear film secreted by goblet cells. We will work with our scientific advisers and seek additional FDA guidance on next steps to designing a Phase 3 trial to confirm OK-101’s novel mechanism of action.”