November 8, 2023
WALTHAM, Mass. — Apellis Pharmaceuticals, Inc. has announced data from the GALE extension study following three years of continuous treatment with SYFOVRE (pegcetacoplan injection), the first-ever FDA-approved treatment for geographic atrophy (GA) secondary to age-related macular degeneration (AMD). The data were reported during an oral presentation at the American Academy of Ophthalmology (AAO) Annual Meeting.
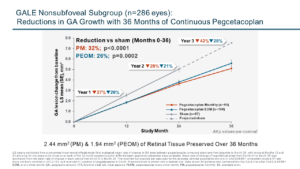
“We are continuing to see increasing SYFOVRE treatment effects year-over-year and a more than 40% reduction in nonsubfoveal lesion growth in the third year,” said Caroline Baumal, MD, chief medical officer, Apellis. “Additionally, SYFOVRE is the only treatment that offers dosing beyond 12 months, and these results demonstrate the importance of early and continuous treatment over time.”
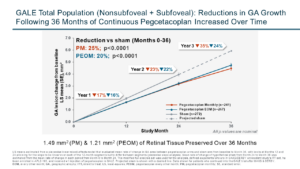
SYFOVRE continued to demonstrate increasing treatment effects over time. In year three, SYFOVRE (all p-values nominal):
- Reduced GA lesion growth with both monthly (35%; p<0.0001) and every-other-month (EOM) (24%; p<0.0001) treatment compared to the projected sham arm.
- Reduced nonsubfoveal GA lesion growth with both monthly (42%; p<0.0001) and EOM (28%; p=0.0015) treatment compared to the projected sham arm.
- Reduced GA lesion growth by 19% (p<0.0001) after one year of SYFOVRE treatment (combined monthly and EOM), compared to the sham treatment period, in patients who crossed over from the sham group.

“These three-year data further solidify the long-term durability of both every-other-month and monthly SYFOVRE, including the increasing treatment effects over time,” said Jeffrey S. Heier, MD, presenting author and director, retina service and director, retinal research, Ophthalmic Consultants of Boston. “GA is a chronic disease that requires long-term management to slow its devastating progression. With the largest dataset collected in GA, I am very encouraged about the potential of SYFOVRE to make a meaningful difference for patients who for so long had no options.”
An analysis of the untreated fellow eye further validated the treatment effects observed in year three. In patients with bilateral GA, lesions are known to grow at similar rates in both eyes. The fellow eye analysis serves as an important additional control to assess treatment effect.
The safety profile of SYFOVRE in year three remained consistent with previously reported data. During the first year of GALE, the rate of new-onset investigator-reported exudative AMD was 7.1% (monthly) and 2.3% (EOM). There was one serious adverse event of ischemic optic neuropathy (ION) in the monthly group between months 24 to 30, which was previously reported, and one case of endophthalmitis between months 30 to 36. The rate of intraocular inflammation (IOI) was 0.26% per injection from months 0 to 36, which does not include the four cases linked to the 2018 impurity. Zero events of retinal vasculitis have been observed in the SYFOVRE clinical trial program, following more than 24,000 injections to date.
Approximately 92% of patients enrolled in GALE completed the first year of the study, demonstrating robust long-term treatment compliance.
About the GALE Long-Term Extension Study
GALE (n=792) is a Phase 3, multicenter, open-label, extension study to evaluate the long-term efficacy and safety of SYFOVRE (pegcetacoplan injection) in patients with GA secondary to AMD. The objectives of the study are to evaluate the long-term incidence and severity of ocular and systemic treatment emergent adverse events as well as change in the total area of GA lesions as measured by fundus autofluorescence. More than 80% of participants who completed the OAKS and DERBY studies entered the GALE study. GALE also includes 10 patients who were previously enrolled in the Phase 1b study of pegcetacoplan for GA.
Patients included in the three-year GALE lesion growth reduction analyses were in the SYFOVRE treatment arms through month 24 in the OAKS and DERBY studies and remained on the same regimen in GALE. Sham-treated patients in the Phase 3 OAKS and DERBY studies were eligible to transition to SYFOVRE treatment in GALE after month 24, so a projected sham arm was used to estimate the growth of GA lesions without treatment between months 24 and 36. The projected sham arm was estimated as the average 12-month mean rate of change in the OAKS and DERBY sham arms through month 24.
About the Phase 3 OAKS and DERBY Studies
OAKS (n=637) and DERBY (n=621) are Phase 3, multicenter, randomized, double-masked, sham-controlled studies comparing the efficacy and safety of SYFOVRE (pegcetacoplan injection) with sham injections across a broad and heterogenous population of patients with GA secondary to AMD. The studies evaluated the efficacy of monthly and every-other-month SYFOVRE in patients with GA assessed by change in the total area of GA lesions from baseline as measured by fundus autofluorescence.
In Phase 3 studies at 24 months, both every-other-month and monthly SYFOVRE reduced GA lesion growth with increasing effects over time and showed a well-demonstrated safety profile.