April 11, 2023
Don’t all eye drops contain water? Until recently, that wasn’t even a question when considering the makeup of clinical topical preparations. Novaliq, a German company, has been quietly changing the very constructs we all hold to be givens when characterizing ophthalmic medications. Kudos to its scientific and strategic teams that have developed the class of semifluorinated alkanes. That sounds a little scary, right? It’s like something you might see printed on the side of a train car labeled “toxic.”
Fortunately, semifluorinated alkanes (SFAs) are considered as diblock molecules with fluorocarbon and hydrocarbon segments and are completely safe and effective as topical agents. Unlike perfluorocarbons (PFCs), SFAs have the potential to dissolve several lipophilic or water-insoluble substances. This makes them feasible as new excipients for drug carrier systems for many of our lipophilic ophthalmic drugs. With two upcoming PDUFA dates issued by the FDA for the approval of semifluorinated alkanes containing agents (see below), it is a good time to review the activity of the two SFAs. One contains the SFA as its active ingredient, and the other contains an SFA plus cyclosporine, where the SFA is the inactive ingredient.
Managing Meibomian Gland Dysfunction is Just Getting Started
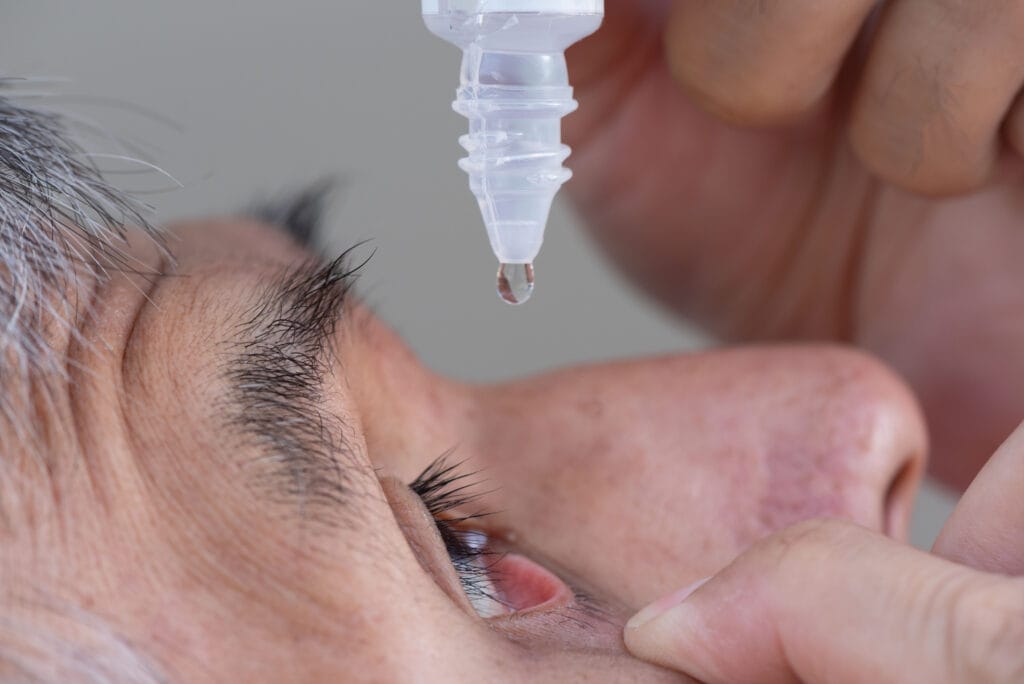
The PDUFA action date is set for June 28, 2023, for NOV03 (perfluorohexyloctane) from Bausch + Lomb. The journey of managing meibomian gland dysfunction (MGD) is seemingly just getting started. During my training in the mid ’90s, MGD just didn’t exist in my textbooks or anterior segment disease courses. You learned there were meibomian glands in the eyelids, but their importance in ocular surface disease was never primary. Fast forward to automated and instrument-assisted heating and evacuation of meibomian glands, and now we have a topical medication specifically for MGD, finally! Bausch + Lomb in-licensed NOV03 from Novaliq GmbH.
NOV03, chemically known as F6H8, is pharmacologically speaking a very different type of agent. There isn’t a vehicle or even a concentration for this drug; it is 100% perfluorohexyloctane. It’s water, steroid, and preservative free. Bausch + Lomb is seeking an indication for excess tear evaporation as well as the signs and symptoms of dry eye disease associated with MGD. The two phase three clinical trials, GOBI and MOJAVE, both met all their primary and secondary symptom and sign endpoints as early as two weeks and through 57 days. The retention time on the ocular surface is approximately 240 minutes. This helps yield a fourfold increase in bioavailability of the drug on the surface and through the cornea.
The chemical cousin of NOV03 or F6H8 is F4H5. This is a distinct chemical moiety from NOV03 or F6H8 but maintains some of the basic tenets of NOV03. It is the carrier for the upcoming FDA approval (PDUFA date June 8, 2023) of CyclAsol, which is 0.1% cyclosporine with the F4H5 as the carrier. One of the great aspects of the entire SFA platform is the ability to take an otherwise very lipophilic drug and allow it to have more bioavailability of the drug on the surface, but more important, it helps facilitate passage of cyclosporine through the cornea.
Drug Innovation is Coming at Breakneck Speed
You may notice that the concentration of cyclosporine at 0.1% is significantly higher than what we see with Restasis/Cequa/generic cyclosporine. It is not even double the concentration once it is on the ocular surface; it is actually less concentration due to F4H5 as the vehicle. When looking at the clinical trial data, I was most impressed with the punctate keratitis data, historically one of the hardest items to satisfy for FDA trials. CyclAsol sets a new bar in terms of speed and mitigation of this clinical enemy. The takeaway for me with this agent is a dual mechanism of action, the traditional cyclosporine effect plus the anti-evaporative effects of the SFAs.
New drug innovation is coming at breakneck speed these days. Expect to see some excitement out of both SFA-based treatments, and I wouldn’t be surprised to see this technology integrated into other therapeutic areas. Glaucoma is one area that could really benefit from such technology, so I’m hopeful that we’ll see some innovation in the way of SFA + PGA in the future.