April 11, 2023
Click here to read “AI and Glaucoma Part II” and here to read “AI and Glaucoma Part III.”
Artificial intelligence (AI) has been in the news with the introduction of ChatGPT, a software program that can create a paragraph when given a sentence or can develop a paper when given a topic.1 Educators are concerned that schools will not be the same. But we have been living with AI for years; it is simply getting better.2 Think about pop-ups on your computer suggesting words as you type a document, items suggested to purchase when you visit the Amazon website, and songs recommended when using Pandora. AI has made it into health care, such as the Apple iWatch monitoring your heart rate for atrial fibrillation or software to detect COVID-19 pneumonia used with chest x-rays.3
Eye care has been slower to incorporate AI into practice, with only one FDA-approved category (screening retinal photographs to detect moderate or worse diabetic retinopathy).4,5 But things will change, enhancing how we take care of our patients.
AI Can Assist with Processing Information to Improve Clinical Decision Making
In our day-to-day practice, we are inundated with information. We vary in how we manage this, but each of us has a similar goal to provide quality eye care. AI relates to the development of devices and software that can do all the things a human can do such as think, reason, and learn. The clinical objective for AI is to aid in processing the abundance of information to allow good clinical decision making, improve quality, enhance patient safety, and ensure we do not inadvertently miss something.
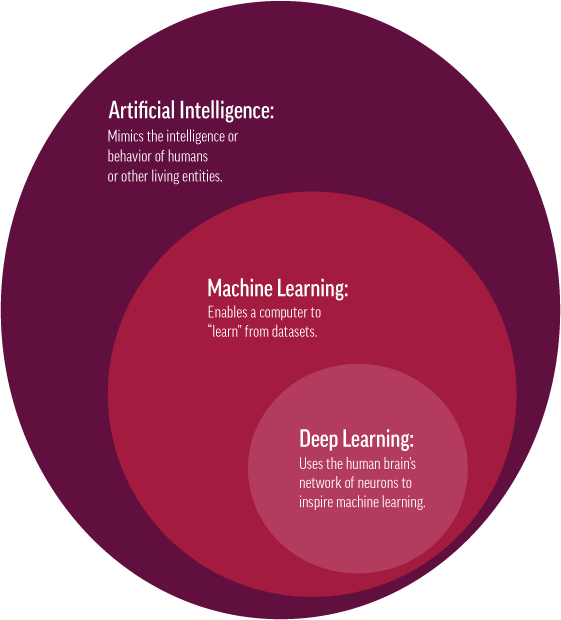
For example, AI used with the OCT should improve segmentation and automate analysis. AI describes a process for software to mimic human thought, allowing it to problem solve as well as self-teach and improve. Deep learning is a subset of AI and machine learning that imitates how the brain processes data, creating patterns useful in decision making.2 It works with neural networks to imitate how humans learn and solve pattern recognition problems without human input. Deep learning can adapt and learn from large amounts of data (“big data”) and identify patterns within images that supplement our clinical judgement.2
Pattern Recognition Laid the Groundwork for AI Analysis
About 20 years ago, the American Academy of Optometry allowed me to put together a glaucoma symposium. For the 2000 meeting, the principal speaker was the noted ophthalmologist, Jost Jonas. Dr. Jonas discussed his approach to assess the optic nerve for glaucomatous damage. As part of this lecture, Dr. Jonas was shown slides he had never seen and was able to predict the patient’s demographic information such as the age, systemic disease status, smoking history. Dr. Jonas explained that he trained himself by examining thousands of images to recognize patterns.
A different example is the sports commentator, Tony Romo, who is known for his ability to predict plays as he calls a football game. His critics remarked that any retired quarterback could do the same thing because being a good observer is what they are trained to do. The information regarding the upcoming play is in a football teams’ formation if one knows what to look for. Similarly, AI can bring information to the clinician’s attention, whether with a retinal picture, visual field printout, or OCT, allowing them to make better decisions.
Glaucoma Diagnosis Requires Sophisticated Analysis of a Variety of Data
Glaucoma is a disease in which AI and deep learning may be useful because the diagnosis and monitoring depend on the analysis of images.6 Also, glaucoma diagnosis is often not obvious and is based on incorporating different pieces of information such as data from intraocular pressure (IOP), corneal thickness, and hysteresis along with fundus and OCT images as well as visual fields printouts. The clinician also processes risk factor information such as family history while there is overlap between so-called “normal” and “abnormal” findings. It is no wonder that a glaucoma diagnosis often requires a sophisticated analysis that could be improved with AI software. AI software could be used with fundus photographs, widefield photographs, OCT, visual fields, anterior segment OCT, and goniophotographs to understand who may be at risk of developing glaucoma or getting worse6.
While we like to think of clinicians as being infallible and rarely missing a finding, this is often not the case. The Glaucomatous Optic Neuropathy Evaluation project (GONE) was developed as a teaching tool and showed how often ophthalmologists and ophthalmology trainees underestimated glaucoma likelihood when assessing the optic discs.7 Also, Jampel et al. showed how difficult it is for glaucoma specialists to detect progressive changes when examining sequential optic disc photographs.8 Diagnosing glaucoma and detecting change, even among experts, is difficult and can be improved. Computers are now faster and more powerful, and with the availability of large datasets, makes the time ripe to develop sophisticated AI programs.
References
1 Stokel-Walker C, AI bot ChatGPT writes smart essays-should professors worry? Nature 2022, December 9. Published online ahead of print.
2 Mukhamediev RI, Popova Y, Kuchin Y, et al. Review of artificial intelligence and machine learning technologies: classification, restrictions, opportunities, and challenges. Mathematics 2022 10; 2552.
3 Salvatore C, Interlenghi M, Monti CB, et al. Artificial intelligence applied to chest x-ray for differential diagnosis of COVID-19 pneumonia. Diagnostics 2021;11:530.
4 Benjamens S, Dhunnoo P, Mesko B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digital Medicine 2020; (3):118.
5 Anton N, Doroftei B, Curteanu S, et al. Comprehensive review on the use of artificial intelligence in ophthalmology and future research directions. Diagnostics (Basel) 2023, 13 (1): 100-123.
6 Ting DSW, Pasquale LR, Peng, L, et al. Artificial Intelligence and deep learning in ophthalmology. Br J Ophthalmology 2019; 103:167-175.
7 O’Neil EC, Pandav SS, Kong YKG, et al. Glaucomatous optic neuropathy evaluation project (GONE): factors associated with underestimation of glaucoma likelihood. JAM Ophthalmol 2014; 132 (5): 560-66.
8 Jampel HD, Friedman D, Quigley H, et al. Agreement among glaucoma specialists in assessing progressive disc changes from photographs in open-angle glaucoma patients. Am J Ophthalmol 2009; 147 (1): 39-44.