April 11, 2023
Click here to read “AI and Glaucoma Part I” and here to read “AI and Glaucoma Part II.”
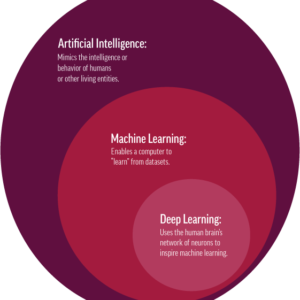
Two studies reflect artificial intelligence’s ability to detect findings not apparent to clinicians.
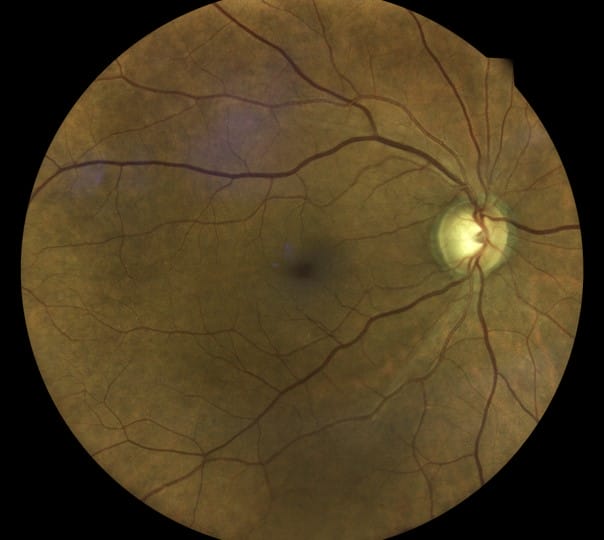
One, Thakur et al., examined data from the Ocular Hypertension Treatment Study (OHTS) with the area under the curve (AUC) for detecting glaucoma four to seven years before development at 0.77 and 0.88 for detecting it one to three years before onset.1 This retrospective study showed that AI analysis may detect signs of progression before the best clinicians observed it.
The second study, Fan et al., also used OHTS data to train a deep learning model based on the retinal photographs from OHTS subjects.2 One objective was to determine if the clinical trial endpoint can be automated using a deep learning model. While there were false positives and negatives, there were cases in which glaucomatous damage was detected before the endpoint committee had recognized the loss. These works illustrate the possibilities of deep learning to enhance our diagnostic skills.
How Will AI be Implemented to Enhance Diagnosis?
Will the screening retinal photography model used for diabetic retinopathy work? That is third party software commercially available that works with a diagnostic device.
In the diabetic retinopathy screening model, which is primarily done in primary care offices, a picture is taken and analyzed by the AI software (cloud-based) with results available within minutes. Would this software be limited to retinal photographs, or could it work with OCT devices since Dicom compatibility standards make using OCT data more complex? Will it be autonomous or assistive (doctors using the AI information to complement their own analysis)?
Will device manufacturers develop AI programs that work with their own devices? Perhaps the companies will use their data management software (such as FORUM from ZEISS or the Topcon Harmony system) to manage the AI analysis.
What About Cost? How Will AI Be Paid For?
While care should improve, will payors reimburse doctors when AI is used? Or will software be part of a device with its cost built into the instrument purchase? Will the device have the computing power and memory to run AI software, or will it all be cloud-based? There are many questions with not many answers.
How will the results be displayed? The FDA approved AI retinal cameras as screening tools, using the software to read the results, which would be flagged if abnormal, leading to a recommendation for further examination. The retinal cameras, OCTs, and perimeters are used for diagnostic indications. Regarding messages the instrument displays, the Humphrey Field Analyzer printout uses terms “possible” or “likely” glaucoma for the Guided Progression Analysis or “within normal limits,” “borderline,” or “outside normal limits” for the Glaucoma Hemifield Test. Will this nomenclature be useful, or will a score be displayed as a continuous variable going from, for example, 0 to 100, with 0 being little risk and 100 being great risk? For these examples, the clinician must analyze the results in context of the other clinical findings. Displaying data is complex, never as simple as it may appear.
The Goal of AI is Not to Replace but to Complement Clinicians
There is no question that AI algorithms can detect a host of retinal conditions using retinal photographs. A recent paper asked: Can AI be more accurate than a clinician? Pandey et al. developed an algorithm to recognize either normal retinas, diabetic retinopathy, glaucoma, or age-related macular degeneration.3 The diagnostic performance of the algorithm was compared against that of ophthalmologists, and AI was found to be more accurate and reliable in classifying these four categories. It shows the possibilities of how care can be improved.
The goal of AI is not to replace clinicians but rather to develop technologies to complement and improve care. In the future, AI software should be available to use with many of our devices to screen for disease, predict and detect disease, predict those at risk for getting worse, and recognize progression.
References
1 Fan R, Bowd C, Christopher M, et al. Detecting glaucoma in the Ocular Hypertension Treatment Study using deep learning. JAMA Ophthalmology 2022; 140(4):383-391.
2 Thakur A, Goldbaum M, Yousefi S. Predicting glaucoma before onset using deep learning. Ophthalmology Glaucoma 2020; 3 (4): 262-268.
3 Pandey PU, Ballios BG, Christakis PG et al. An ensemble of deep convolutional neural networks is more accurate and reliable than board-certified ophthalmologists at detecting multiple disease in retinal fundus photographs. Br J Ophthalmol Jan 2023 online ahead of print