June 7, 2023
“The best time to plant a tree was 20 years ago, the second best time is today”
— Chinese proverb
Diabetic retinopathy represents a leading cause of vision loss globally, and the time to act is before damage occurs. Insulin resistance and prediabetes insidiously occur, often years before a diagnosis of diabetes. Affecting more than one in three adults in America, prediabetes is defined as a fasting blood glucose range of 100-125 mg/dl or a hemoglobin A1c between 5.7-6.4%.1
The CDC’s Division of Diabetes Translation has released the National Diabetes Statistics Report, which presents the most current statistics on the “state of the disease” in the United States. The percentage of adults with prediabetes who were aware they had the condition nearly tripled between 2005 and 2020, but most continue to be unaware.
Knowledge is power. As eye care practitioners, we evaluate the health of the vasculature system with each retinal exam. Emerging research into the power of lifestyle interventions to mitigate the risk and course of type 2 diabetes enables us to start the dialogue with our patients sooner now than ever before, helping to preserve vision in later years.
Diabetic Retinopathy: An Insidious Thief of Sight
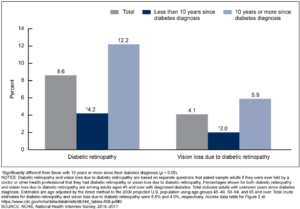
Diabetic retinopathy (DR) is a major microvascular complication of diabetes and a leading cause of vision loss and blindness globally.2 Nearly all patients with type 1 and >60% of patients with type 2 diabetes (T2D) will have some form of DR within 20 years of developing diabetes.3 The longer a patient has diabetes, the greater the risk of diabetic retinopathy over a lifespan. In a National Health Interview Survey 2016-2017 by the CDC, among adults aged 45 and over with diagnosed diabetes, 8.6% had diabetic retinopathy, and rates of vision loss increased significantly in those who had the disease for 10 years or more. With the rising numbers of younger people with insulin resistance and diabetes, rates of DR will be increasing in the future among patients at a much higher rate.4 In fact, in a CDC report released December 2019, nearly 1 in 5 (18%) of adolescents (ages 12 to 18) and 1 in 4 (24%) of young adults (ages 19 to 34) were found to be living with prediabetes. Increasing rates in younger demographics will mean that the prevalence of diabetic retinopathy in those over 45 will continue to increase in the future.
Figure 1. Age-adjusted percentage of adults aged 45 and over with diagnosed diabetes who had diabetic retinopathy and vision loss due to diabetic retinopathy, by years since diabetes diagnosis: United States, 2016-2017
Dietary Patterns and Reduced DR risk
Hyperglycemia is an important factor in the pathogenesis of the microvascular complications that are at the root of damage from diabetes, but other factors appear to be involved as well. Certain metabolic abnormalities and pathological changes that are associated with diabetes might be at least partly independent of blood glucose levels. A growing body of evidence suggests that oxidative stress and inflammation play an integral role in the early pathogenesis of diabetic retinopathy by potentiating retinal neurodegeneration.5 The onset of T2D mellitus (T2DM) starts with prediabetes and insulin resistance leading to insulin deficiency, hyperglycemia, and dyslipidemia. This in turn enhances the pro-oxidant and pro-inflammatory pathways that are at the root of most chronic diseases of aging.
Concentrating on dietary profiles rather than specific nutrients is proving to be more beneficial due to the synergistic effects of the many different nutritional components in an entire diet, rather than selected supplementation of micronutrients. Anti-inflammatory eating patterns can mitigate the oxidative stress and inflammation that are elevated in type 2 diabetes.
In 2018, Wong et al. conducted a systematic review of dietary patterns and DR that showed higher intakes of dietary fiber, fruits and vegetables, oily fish, and a greater adherence to a Mediterranean diet were protective of DR.6 Conversely, high total caloric intake was associated with a higher risk of DR. The ingestion of dietary fiber tends to modulate the postprandial glucose response and is, thus, proposed to reduce glucose-induced damage to the retina.6
Kadri et al. observed an inverse relationship between more frequent (>2 times/week) fish consumption and DR progression.7 Two other large studies, one on Spanish adults and one on an Asian population, also showed that at least two servings of oily fish a week had a significantly lower risk of DR.8,9 This is consistent with evidence that shows that fatty fish two times a week is also protective against age-related macular degeneration.
The findings of these studies support existing American Diabetic Association guidelines for overall diabetes management that acknowledge the beneficial effects of a Mediterranean diet, encouraging people with diabetes to eat a diet rich in fruits and vegetables and consume fewer calories.10
Specific Nutrients Support Retinal Health in Diabetes
The following nutrients, by their natural physiological, biochemical, and molecular action, can preserve retinal structure and function by interfering with the various pathological steps that promote DR. In addition to recommending an anti-inflammatory Mediterranean diet, practitioners might want to consider the following in their recommendations.
Vitamin D
Vitamin D is a fat-soluble vitamin that functions as a pro-hormone. It is associated with over 200 functions in the body. Research supports the role of vitamin D in DR, making its deficiency a risk factor. The prevalence of vitamin D deficiency in the United States is above 40%, and it is higher in the elderly and in some ethnic groups.11 Many patients over 55 years of age would benefit from supplementation. It is a potent inhibitor of angiogenesis, and its angiogenic effect may be mediated by vitamin D receptors in the retina.12,13
Vitamin D sufficiency is essential for insulin release, insulin sensitivity, reduction of inflammation, and reduction of arterial stiffness.14, 15, 16, 17, 18, 19, 20 Recently, optimal vitamin D levels are shown to reduce the risk and severity of DR.21 Vitamin D also plays a role in pancreatic β-cell function.22,23 Deficiency reduces insulin sensitivity and increases the risk of atherosclerosis, CVD, T2DM, and hypertension.23, 24, 25,26
By stimulating pancreatic beta cells, 1,25–hydroxy vitamin D triggers the secretion of insulin.19, 20 Clinical trials show significant improvements in insulin sensitivity and HbA1c when patients were given vitamin D3.26, 27 Mutlu et al. reports that lower vitamin D was associated with retinal microvascular damage after studying the associations in 5,675 participants who have diabetes.28
It is well established that vitamin D deficiency is linked to T2DM. Supplementation with D3 has also been shown to decrease inflammation by lowering C-reactive protein overall and hs-CRP levels.29 Serum levels of 25-hydroxy vitamin D above 30 ng/ml reduce the odds of DR.21, 30 Vitamin D supplementation reduces intracellular reactive oxygen species, decreasing VEGF expression.31 Low serum vitamin D levels among patients with DM are associated with a higher risk and severity of DR for all the above reasons. Cumulatively, this suggests that vitamin D supplementation is beneficial to reduce the risk and severity of DR.32 Because vitamin D is a fat-soluble vitamin, patients should check with their physician for baseline testing before supplementing with high doses.
Omega 3 Fatty Acids
Evidence shows that omega-3s can also inhibit the development and progression of DR.33, 34 ,35, 36 Omega-3 fatty acid metabolites, such as lipoxins, resolvins, and protectins, exhibit anti-inflammatory action by suppressing interleukin (IL)-6, tumor necrosis factor (TNF)-alpha, VEGF, and reactive oxygen species (ROS), and restore antioxidant homeostasis.37
Sala-Vila et al. examined this association in humans by conducting a long-term prospective study of 3,482 patients with T2D (mean age, 67 years; 48% men) enrolled in the PREDIMED trial. They found that consumption of omega-3 fatty acids (≥500 mg/d) significantly reduced the risk of DR.38 The relative risk of incident sight-threatening DR for these participants was 46% lower than for those who consumed less omega 3s (p = 0.001), regardless of the assigned diet. Risk reduction was even greater for patients with hypertension or advanced diabetes. Participants who consumed at least two servings of oily fish per week at baseline also had a lower risk of DR.38 Omega 3s, in particular DHA, has been shown to increase the production of brain-derived neurotrophic factor (BDNF), which can protect retinal neuronal cells from degeneration caused from DR.39
The omega 3 polyunsaturated fatty acids EPA and DHA prevent IL-6 and TNF-alpha production and suppress intercellular cell adhesion molecule expression and VEGF secretion.39 High-glucose-induced retinal vascular endothelial damage is reduced by omega-3s as well.39 It must be noted that not all studies consistently show a benefit of omega-3 supplementation in DR, however, an overall pattern is emerging that a diet high in oily fish (i.e., two servings per week) is protective for not only DR but also other diseases of the retina, such as AMD.38
Carotenoids
For well over two decades, the carotenoids lutein, zeaxanthin, and meso-zeaxanthin have been studied for their ability to mitigate oxidative stress in macular degeneration. Lem et al.40 make an argument that decreased levels and depletion of the endogenous antioxidant defense system in the retina from diabetes can also be sufficiently augmented via carotenoid vitamin therapy. They propose using macular pigment optical density measurements as an early biomarker of oxidative stress in the retina.
Supplementing with carotenoids for DR is not a new concept. The carotenoids lutein, zeaxanthin, and meso-zeaxanthin are shown to have antioxidant effects, reduce vascular permeability and leakage, and maintain blood vessel integrity.41 In some studies, they are shown to reduce the risk of diabetic retinopathy pathogenesis.41, 42, 43 Brazionis et al. report that, similar to AMD, higher lutein and zeaxanthin levels were associated with significantly lower odds of DR.42 A two-year trial of 10 mg lutein plus 12 mg zeaxanthin in diabetic patients without DR showed improved retinal response density on multifocal electroretinography and a mild non-edematous increase in foveal thickness.44 Evidence also suggests that lutein supplementation increases brain-derived neurotrophic factor (BDNF), preventing neurodegeneration, and preserving electroretinograms.45, 46

Alpha Lipoic Acid (ALA)
Alpha lipoic acid is a thiol antioxidant that is synthesized by humans and also occurs in small amounts in a wide range of foods. It functions as a cofactor for certain mitochondrial enzymes, has antioxidant properties, and promotes the regeneration of other antioxidants such as vitamin E, vitamin C, and glutathione. Some research suggests that ALA supplementation may help improve visual function and reduce oxidative stress in the retina.47 Supplementation with 600 mg/day of ALA has been reported to increase insulin sensitivity in patients with type 2 diabetes.48 In a randomized trial, supplementation with 300 mg/day of ALA for three months improved or prevented the deterioration of contrast sensitivity in patients with type 1 and type 2 diabetes.49 ALA has been shown to reduce both small and large blood vessel complications of diabetes in animal models.50 ALA treatment reduced retinal capillary damage, oxidative stress, nuclear factor kappa-beta activation and VEGF in an animal model of diabetic retinopathy.51 More studies are needed on the effects of ALA for the prevention of diabetic retinopathy, but ALA’s ability to mitigate oxidative stress and decrease inflammation in the body is well established in the literature. Typical doses range from 200 to 600 mg/day in divided doses for larger amounts.
Dietary Intake and Risk of Diabetic Retinopathy
The evidence linking dietary intake and diabetic retinopathy is growing. With more research dollars being allotted to study the relevance of dietary patterns and disease, our knowledge will continue to rapidly expand. Targeting “inflammaging” through evidence-based diet and lifestyle recommendations allows practitioners to address the commonality that links all chronic diseases of aging that is chronic, sustained inflammation and oxidative stress.
In Summary:
- Prediabetes affects one in three American adults, and lifestyle modifications such as dietary changes have been shown to reduce the risk of development of type 2 diabetes.
- Diabetic retinopathy represents a leading cause of vision loss globally, and the time to act is before damage occurs.
- A Mediterranean style diet with fatty fish two times per week has been shown to reduce the risk of diabetic retinopathy.
- Vitamin D deficiency is a risk factor for diabetic retinopathy, and many patients over 55 years of age would benefit from supplementation.
- Omega 3 fatty acids, through multiple mechanisms, have been shown in studies to mitigate the risk of diabetic retinopathy.
- Carotenoids such as lutein, zeaxanthin, and mezo-zeaxanthin can decrease oxidative stress and inflammation in the retina and protect against diabetic retinopathy.
- Alpha lipoic acid is a strong antioxidant that has been shown in studies to combat oxidative stress and protect retinal tissues, but more research is needed.
References
1 About Prediabetes & Type 2 Diabetes. Centers for Disease Control and Prevention.(2022).
2 Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298(8):902-916.
3 Fong DS, Aiello L, Gardner TW, et al. Retinopathy in diabetes. Diabetes Care. 2004;27 Suppl 1:S84-7.
4 Bai P, Barkmeier AJ, Hodge DO, Mohney BG. Ocular Sequelae in a Population-Based Cohort of Youth Diagnosed With Diabetes During a 50-Year Period. JAMA Ophthalmol. 2022;140(1):51-57.
5 Lem DW, Gierhart D L, Davey PG. Management of Diabetic Eye Disease Using Carotenoids and Nutrients. In (Ed.), Antioxidants – Benefits, Sources, Mechanisms of Action. IntechOpen. 2021.
6 Wong MY, Man RE, Fenwick EK, et al. Dietary intake and diabetic retinopathy: A systematic review. PLoS One. 2018;13(1):e0186582.
7 Kadri R, Vishwanath P, Parameshwar D, et al. Dietary associations with diabetic retinopathy- A cohort study. Indian J Ophthalmol 2021;69(3):661-665.
8 Chua J, Chia AR, Chee ML, Man REK, Tan GSW, Lamoureux EL, Wong TY, Chong MF, Schmetterer L. The relationship of dietary fish intake to diabetic retinopathy and retinal vascular caliber in patients with type 2 diabetes. Sci Rep. 2018 Jan 15;8(1):730. doi: 10.1038/s41598-017-18930-6. PMID: 29335432; PMCID: PMC5768794.
9 Sala-Vila A, Díaz-López A, Valls-Pedret C, et al. Dietary Marine ω-3 Fatty Acids and Incident Sight-Threatening Retinopathy in Middle-Aged and Older Individuals With Type 2 Diabetes: Prospective Investigation From the PREDIMED Trial. JAMA Ophthalmol. 2016;134:1142–1149. doi:10.1001/jamaophthalmol.2016.2906
10 American Diabetes Association. Standards of Medical Care in Diabetes-2013. Diabetes Care.2013;36:S11-S66.
11 Shi C, Want P, Airen S, et al. Nutritional and medical food therapies for diabetic retinopathy. Eye and Vision. 2020;7:33.
12 Sharma Y, Saxena S, Mishra A, et al. Nutrition for diabetic retinopathy: plummeting the inevitable threat of diabetic vision loss. Eur J Nutr. 2017;56:2013-2027.
13 Taverna MJ, Selma JL, Slama G. Association between a protein polymorphism in the start codon of the vitamin D receptor gene and severe diabetic retinopathy in C-peptide-negative type 1 diabetes. J Clin Endocrinol Metab 2005;90:4803-4808.
14 Vitamin D-Health Professional Fact Sheet. National Institutes of Health. 2020. Accessed 24 Mar 2020.
15 Perez-Lopez FR. Vitamin D: the secosteroid hormone and human reproduction. Gynecol Endocrinol. 2007;23(1):13–24.
16 Carvalho JTG, Schneider M, Cuppari L, et al. Cholecalciferol decreases inflammation and improves vitamin D regulatory enzymes in lymphocytes in the uremic environment: A randomized controlled pilot trial. PLoS One. 2017;12(6):e0179540.
17 Dong Y, Stallmann-Jorgensen IS, Pollock NK, et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab. 2010;95(10):4584–4591.
18 Witham MD, Dove FJ, Dryburgh M, et al. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2010;53(10):2112–2119.
19 Sergeev IN, Rhoten WB. 1,25-Dihydroxyvitamin D3 evokes oscillations of intracellular calcium in a pancreatic beta-cell line. Endocrinology. 1995;136(7):2852–2861.
20 Kramer CK, Swaminathan B, Hanley AJ, et al. Prospective associations of vitamin D status with beta-cell function, insulin sensitivity, and glycemia: the impact of parathyroid hormone status. Diabetes. 2014;63:3868–3879.
21 Long M, Wang C, Liu D. Glycated hemoglobin A1C and vitamin D and their association with diabetic retinopathy severity. Nutr Diabetes. 2017;7(6):e281.
22 Rashidi B, Hoseini Z, Sahebkar A, Mirzaei H. Anti-Atherosclerotic Effects of Vitamins D and E in Suppression of Atherogenesis. J Cell Physiol. 2017;232(11):2968–2976.
23] Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5):820–825.
24 Bonakdaran S, Rokni H. Diabetic CVD–focus on vitamin D. Cardiovasc Hematol Agents Med Chem. 2012;10(3):241–250.
25 Boucher BJ. Vitamin D insufficiency and diabetes risks. Curr Drug Targets. 2011;12(1):61–87.
26 Li X, Liu Y, Zheng Y, Wang P, Zhang Y. The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Nutrients. 2018;10(3):375.
27 Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complications. 2017;31(7):1115–1126.
28 Mutlu U, Ikram MA, Hofman A, et al. Vitamin D and retinal microvascular damage: The Rotterdam Study. Medicine (Baltimore). 2016;95(49):e5477.
29 Yu Y, Tian L, Xiao Y, Huang G, Zhang M. Effect of vitamin D supplementation on some inflammatory biomarkers in type 2 diabetes mellitus subjects: a systematic review and meta-analysis of randomized controlled trials. Ann Nutr Metab. 2018;73(1):62–73.
30 Millen AE, Sahli MW, Nie J, et al. Adequate vitamin D status is associated with the reduced odds of prevalent diabetic retinopathy in African Americans and Caucasians. Cardiovasc Diabetol. 2016; 15(1):128.
31 Lu L, Lu Q, Chen W, et al. Vitamin D3 protects against diabetic retinopathy by inhibiting high-glucose-induced activation of the ROS/ TXNIP/NLRP3 inflammasome pathway. J Diabetes Res. 2018;2018:8193523.
32 Shi C, Want P, Airen S, et al. Nutritional and medical food therapies for diabetic retinopathy. Eye and Vision. 2020;7:33.
33 Tikhonenko M, Lydic TA, Opreanu M, et al. N-3 polyunsaturated fatty acids prevent diabetic retinopathy by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function. PLoS One 8(1):e55177.
34 Sapieha P, Chen J, Stahl A, et al. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutr Diabetes. 2012;2:e36.
35 Koto T, Nagai N, Mochimaru H, et al. Eicosapentaenoic acid is anti-inflammatory in preventing choroidal neovascularization in mice. Invest Ophthalmol Vis Sci. 2007;48(9):4328-4334.
36 Howard-Williams J, Patel P, Jelfs R, et al. Polyunsaturated fatty acids and diabetic retinopathy. Br J Ophthalmol. 1985;69:15-18.
37 Sharma Y, Saxena S, Mishra A, et al. Nutrition for diabetic retinopathy: plummeting the inevitable threat of diabetic vision loss. Eur J Nutr. 2017;56:2013-2027.
38 Sala-Vila A, Díaz-López A, Valls-Pedret C, et al. Dietary Marine ω-3 Fatty Acids and Incident Sight-Threatening Retinopathy in Middle-Aged and Older Individuals With Type 2 Diabetes: Prospective Investigation From the PREDIMED Trial. JAMA Ophthalmol. 2016;134:1142–1149. doi:10.1001/jamaophthalmol.2016.2906
39 Shen J, Bi YL, Das UN. Potential role of polyunsaturated fatty acids in diabetic retinopathy. Arch Med Sci. 2014;10(6):1167-1174.
40 Lem DW, Gierhart D L, Davey PG. Management of Diabetic Eye Disease Using Carotenoids and Nutrients. In (Ed.), Antioxidants – Benefits, Sources, Mechanisms of Action. IntechOpen. 2021.
41 Hu BJ, Hu YN, Lin S, Ma WJ, Li XR. Application of lutein and zeaxanthin in nonproliferative diabetic retinopathy. Int J Ophthalmol. 2011;4(3):303-306.
42 Brazionis L, Rowley K, Itsiopoulos C, O’Dea K. Plasma carotenoids and diabetic retinopathy. Br J Nutr. 2009;101:270-277.
43 Tanaka S, Yoshimura Y, Kawasaki R, Kamada C, Tanaka S, Horikawa C, Ohashi Y, Araki A, Ito H, Akanuma Y, Yamada N, Yamashita H, Sone H; Japan Diabetes Complications Study Group. Fruit intake and incident diabetic retinopathy with type 2 diabetes. Epidemiology. 2013 Mar;24(2):204-11. doi: 10.1097/EDE.0b013e318281725e. PMID: 23348071.
44 Moschos MM, Dettoraki M, Tsatsos M, Kitsos G, Kalogeropoulos C. Effect of carotenoids dietary supplementation on macular function in diabetic patients. Eye Vis (Lond). 2017;4:23.
45 Sasaki M, Ozawa Y, Kurihara T, et al. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010;53(5):971–979.
46 Li SY, Fung FK, Fu ZJ, Wong D, Chan HH, Lo AC. Anti-inflammatory effects of lutein in retinal ischemic/hypoxic injury: in vivo and in vitro studies. Invest Ophthalmol Vis Sci. 2012;53(10):5976–5984.
47 Ajith TA. Alpha-lipoic acid: A possible pharmacological agent for treating dry eye disease and retinopathy in diabetes. Clin Exp Pharmacol Physiol. 2020 Dec;47(12):1883-1890. doi: 10.1111/1440-1681.13373. Epub 2020 Jul 21. PMID: 32621549.
48 Jacob S, Ruus P, Hermann R et al, Oral administration of rac-alpha lipoic acid modulates insulin sensitivity in patients with type 2 diabetes mellitus: a placebo controlled pilot trial. Free Radic Biol Med 1999;27:309-314.
49 Gebka A, Serkies-Minuth E, Raczynska D. Effect of the administration of alpha lipoic acid on contrast sensitivity in patients with type 1 and type 2 diabetes. Mediators Inflamm 2014;2014:131538.
50 Lin J, Bierhaus A, Bugert P, et al. Effect of R-alpha-lipoic acid on experimental diabetic retinopathy. Diabetologia. 2006 May;49(5):1089-96.
51 Voloboueva LA, Liu J, Suh JH. R-alpha-lipoic acid protects retinal pigment epithelial cells from oxidative damage. Invest Ophthalmol Vis Sci. 2005 Nov;46(11):4302-10.