April 18, 2023
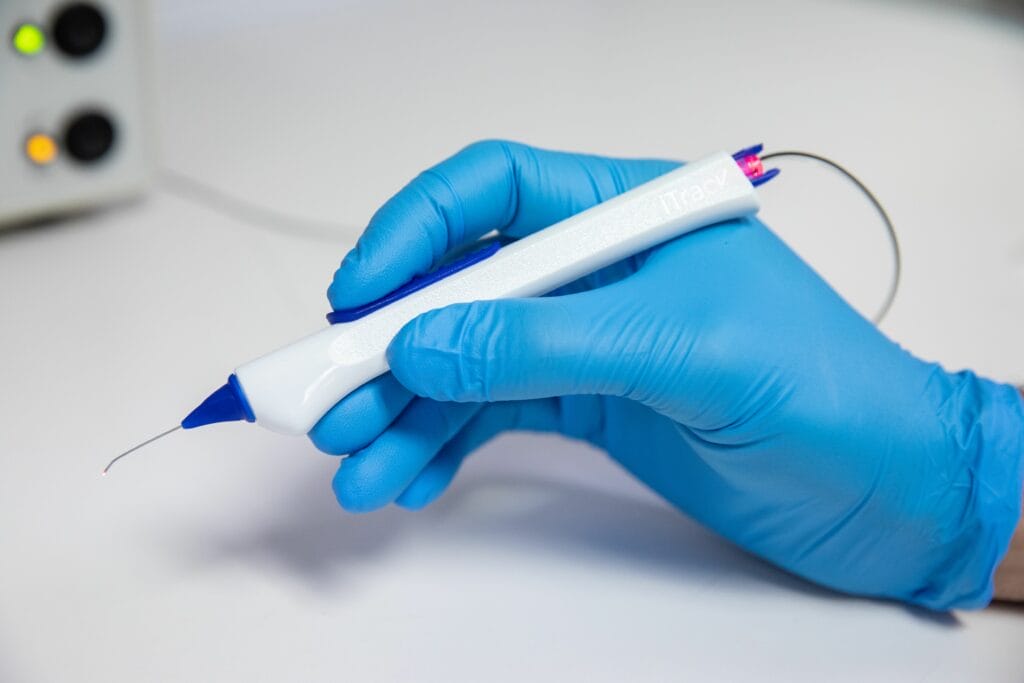
FREMONT, Calif. — The U.S. Food and Drug Administration has granted 510(k) clearance to Nova Eye Medical Limited’s new canaloplasty device, iTrack Advance. The newest generation canaloplasty device for canal-based glaucoma surgery will be available in May 2023 to surgeons in the U.S. to treat glaucoma.
The original iTrack was the pioneering canaloplasty device that first established canal surgery for glaucoma, and to date approximately 120,000 surgeries have been performed by surgeons worldwide. The iTrack microcatheter is the only product that is indicated for canal surgery to treat glaucoma with viscodilation alone.
iTrack Advance is the latest addition to the iTrack family and is a high precision hand-held delivery system that places the clinically proven iTrack microcatheter into the main drainage canal of the eye for injection of viscoelastic fluid (canaloplasty) to clear blockages that cause elevated eye pressure (glaucoma). The original iTrack is principally used by glaucoma surgeons, whereas the new iTrack Advance, with the extended feature set, is expected to appeal to glaucoma surgeons as well as cataract surgeons and comprehensive surgeons, for use in a combination procedure alongside cataract surgery. iTrack Advance can be used by surgeons for both standalone procedures as well as in combination with cataract surgery.
Canaloplasty was first introduced into the U.S. market in 2008, following the release of the company’s original iTrack canaloplasty microcatheter. Dr. Mahmoud A. Khaimi, a prominent canaloplasty surgeon, was the first surgeon in the U.S. to perform canaloplasty with the new iTrack Advance. The surgeries were performed at the world-renowned Dean McGee Eye Institute. The company also benefitted from the expertise of Clinical Professor, James P. Luton, MD, Endowed Chair in Ophthalmology at Dean McGee Eye Institute, University of Oklahoma.
“I’ve been given the great opportunity to pair hand in hand with Nova Eye Medical to develop the iTrack Advance. We’ve taken the original iTrack canaloplasty microcatheter and teamed it with an ergonomic handpiece that facilitates improved access into the canal,” said Dr. Khaimi. “Thanks to the handpiece, we can advance the microcatheter and then retract it along the full circumference of Schlemm’s canal with much greater efficiency than ever before.”
Dr. Khaimi added that surgeons will continue to benefit from Nova Eye’s proprietary illuminated microcatheter technology. It debuted with the original iTrack, and now with the iTrack Advance, is the world’s only illuminated canaloplasty microcatheter. In the U.S, the iTrack Advance has been cleared for canaloplasty both with and without concurrent cataract surgery.
The iTrack Advance will be officially launched in the U.S. at the American Society of Cataract and Refractive Surgery (ASCRS) in San Diego, May 5-8, 2023. This device has been cleared for use since June 2022 throughout Canada, Australia, and Europe, including Germany, where a multi-center, randomized study (“CATALYST”, CTN: NCT05564091) is currently underway to evaluate the effectiveness of canaloplasty with the iTrack Advance performed in combination with cataract surgery, as compared to cataract surgery alone. The CATALYST study is expected to reinforce the clinical utility of canaloplasty in the treatment of mild to moderate glaucoma patients.