September 27, 2023
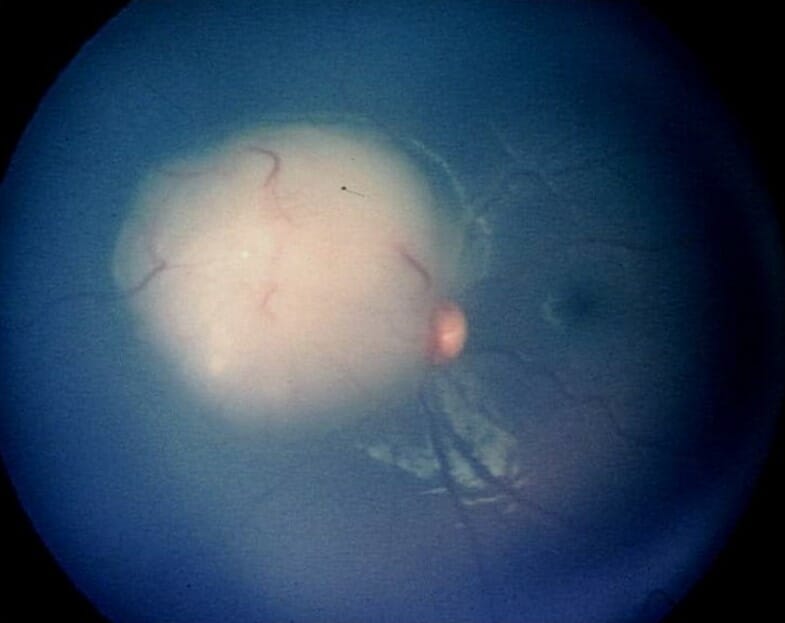
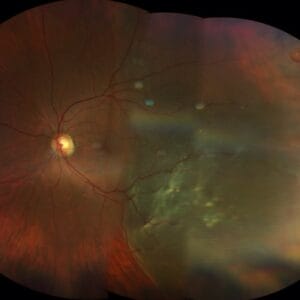
It has probably been quite a while since you’ve given much thought to retinoblastoma (RB). After all it is quite rare, and as optometrists we’re not likely to be much involved in the direct management of RB beyond recognition and ensuring these children are in their best optical correction while they are undergoing treatment, especially early when vision development is so critical. No doubt as optometrists we may be the first point of contact to discover a child who is suspected of having RB. It is well known that RB is the most common intraocular malignancy in children. One of the hallmarks of RB is leukocoria, so it becomes critically important as an eye care provider to make sure an infant who presents with a white reflex doesn’t have RB.
So, what happens to these young children once they leave our offices and end up in the hands of an ocular oncologist? The evolution in the treatment of RB over the past two decades has been quite remarkable. Even though the focus of Review of Presbyopia and the Aging Eye is to address issues relating to the aging eye, the fact is that today almost all these kids will now survive and grow up having both eyes. Therefore, they will need to deal with the common issues that we see in presbyopia and the aging eye. So, let’s go back to the beginning and see how our understanding of this interesting disease has changed and more important how treatments have evolved to make RB the most curable of all pediatric cancers with a survival rate that exceeds 99%,1,2 allowing these kids to grow up to eventually become aging presbyopes.
Initial RB Treatment Involved Eye Removal
Through most of the 20th century, enucleation was standard treatment of RB, especially for bilateral RB, with the hope that removing the eye and thus the cancer, would increase the chance for survival. By the late 1980s and into the 1990s, external beam radiation evolved as a conservative globe-sparing approach, especially in bilateral RB. It became widely successful as a cure and emerged as one of the only malignancies in children that can be cured by radiation alone. Unfortunately, as many children survived and were able to keep their eye, almost none were able to retain any useful vision.
What’s more, many of these children went on to develop second (and third) malignancies, particularly in the area that was radiated, and over 50% of patients would die by age 40 from second non-ocular malignancies.3,4 This was only true for the hereditary, germline mutations, suggesting that these patients may have a genetic predisposition for developing malignancies, which turned out to be the case.
The retinoblastoma gene is a tumor-suppressing gene that works to inhibit not only the development of RB but other cancers as well. This tumor-suppressing gene is present in every cell in our body, so patients who have an abnormality with this gene are susceptible to the development of other cancers, not just RB. For some reason, these non-ocular malignancies were more likely to develop in an area that had already undergone radiation and even more so if the radiotherapy was done before 12 months of age. By the 1990s, the most common cause of death in RB patients was not from the RB but from secondary cancers or as a result of the radiation treatment.4
The increased risk for second malignancies resulted in a shift away from radiation to systemic chemotherapy, which was highly effective in eyes with less advanced tumors but proved much more difficult in advance cases. This was especially true in the presence of vitreous or subretinal seeding, which accounts for the majority of RB cases worldwide. As good as it was, it did not cure the cancer, so many patients needed additional localized treatments to the tumors, including radioactive plaque, laser photocoagulation, cryotherapy, and even enucleation. Unfortunately, with chemotherapy, the entire drug dose enters the systemic circulation, but only a small fraction becomes available at the site of the ocular tumor, so systemic toxicity from the chemotherapy was significant. Hearing loss from ototoxicity was seen in as many as one-third of cases. Other complications included myelocytic anemia, a virulent form of leukemia, among other complications. There were also localized issues related to infusion ports and the need for transfusions.2
Intra-arterial Chemotherapy Saves Lives and Eyes
As a result of these issues, a more targeted approach was developed. Beginning in 2006, David Abramson pioneered a superselective intra-arterial delivery of chemotherapy to the ophthalmic artery using a microcatheter. The catheter is inserted into the femoral artery and then passed through the abdominal and thoracic aorta and into the internal carotid artery, where it is then placed directly in the ophthalmic artery. There are some variations on the approach to the ophthalmic artery based on the child’s unique anatomy, but the end result is infusion of a small volume of a chemotherapeutic agent (or agents) into the ophthalmic artery — between 0.5 and 1 cc — over a course of 30 minutes. It delivers a high concentration of chemotherapy to the eye at a dose that is 100 times more concentrated than if the same concentration was delivered systemically. 5 Patients, on average, require approximately three infusions.
With this form of localized treatment, these children do not get sick because the systemic dose is so small, nor do they lose their hair or suffer any significant systemic affects. Most important, this treatment has resulted in a 99% survival of RB. It has reduced the need for enucleation in over 95% of cases and reduced the risk of metastasis to less than 1%. 2,3,5 What’s more, it is more effective, faster, easier on the child and the family, and less expensive. This superselective ophthalmic artery delivery has revolutionized the treatment for RB, and it is now widely used around the world.
Not only are these children surviving, but many end up with pretty good visual outcomes. In a series of 54 eyes with RB treated by intra-arterial chemoreduction, 50% of eyes at five years were able to see 20/40 or better, and 67% were able to see 20/200 or better. Of those with bilateral RB, 75% had a final visual acuity of 20/40 or better in at least one eye.6 In another series by Narang et al. of 140 eyes following chemoreduction for retinoblastoma, 37% had a visual acuity of 20/40, and 71% achieved a visual acuity 20/200 or better.7 The limiting factor for better visual acuity in both series was the presence of macular or extramacular lesions. When the RB lesions were outside the macula, over 90% were 20/40 or better.
The success story of retinoblastoma in the era of modern medicine is truly remarkable. If you take a step back and consider the idea of inserting a microcatheter into the femoral artery on an infant or young child and running it all the way up through the internal carotid artery to the ophthalmic artery to deliver a localized treatment into the eye, it seems like space-age technology, but it isn’t any more. It has evolved to become the standard of care for treating RB all around the world, giving it the highest cure rate of any pediatric tumor. As a result, these children will grow up to become aging presbyopes dealing with the same issues that we all do: needing reading glasses, dealing with dry eyes, developing cataracts, and a host of other things that we as primary eye care providers are very familiar and deal with every day. Hopefully, the days of these children missing an eye will come to an end, and the success of RB can be a template for survival for other cancers.
References
1 Group GRS, Fabian ID, Abdallah E, Abdullahi SU, Abdulqader RA, Boubacar SA, et al. Global Retinoblastoma Presentation and Analysis by National Income Level. JAMA Oncol (2020) 6:685–95. doi:
2 Schaiquevich P, Francis, JH, Cancela MG, et al. Treatment of retinoblastoma: what is the latest and what is the future. Frontiers in Oncology. 2022;12:822330.
3 Abramson DH, Schefler AC, Update on retinoblastoma. Retina. 2004; 24:828-848.
4 Abramson DH, Melson MR, Dunkel IJ, et al. Third (fourth and fifth) nonocular tumors in survivors of retinoblastoma. Ophthalmology. 2001 Oct;108(10):1868-76. doi: 10.1016/s0161-6420(01)00713-8
5 Abramson DH. The evolution of treatments for retinoblastoma. Retina Today. Nov/Dec 2010; 56-58.
6 Demirci H, Shields CL, Meadows AT, Shields JA. Long-term visual outcome following chemoreduction for retinoblastoma. Arch Ophthalmol. 2005;123:1525-1530.
7 Narang MS, Mashayekhi A, Rudich D, Shields CL. Predictors of long-term visual outcome after chemoreduction for management of intraocular retinoblastoma. Clin Experiment Ophthalmol. 2012:40(7):736-742.