February 2, 2024
The macular pigment carotenoids, mainly lutein, zeaxanthin, and meso-zeaxanthin, are xanthophylls that uniquely accumulate in the macula, the central part of the retina responsible for sharp, detailed vision.1, 2 The macular pigment, obtained exclusively through diet or supplementation, functions primarily as a natural filter, absorbing and neutralizing harmful high-energy blue light that enters the eye and scavenges free radicals via its antioxidant properties.3-5 This protective mechanism helps shield the delicate retinal cells from oxidative damage, which is particularly important for preventing age-related macular degeneration (AMD). AMD is a leading cause of irreversible vision loss, and low levels of macular pigment are associated with an increased risk of developing this debilitating condition.6
The significance of macular pigment in eye health extends beyond its role as a protective short-wavelength filter. Research has shown that adequate macular pigment levels are linked to enhanced visual and cognitive performance.7, 8 Thus, individuals with higher macular pigment density often experience improved contrast sensitivity and reduced sensitivity to glare.9, 10 These factors contribute to better overall visual function, particularly in challenging conditions such as low-light environments or when exposed to bright light sources. The importance of maintaining optimal macular pigment levels underscores the role of nutrition in supporting ocular health.
Given the importance of macular pigment in eye health, assessing macular pigment status in routine clinical examinations could benefit proactive eye care and disease prevention by providing valuable insights into an individual’s overall ocular health and visual performance. Thus, early detection of insufficient macular pigment status allows for targeted interventions, such as dietary adjustments or supplementation, to enhance the protective function of the macula and potentially mitigate the risk of developing AMD, enhancing the quality of life for individuals as they age.
Overview of Macular Pigment Assessment Modalities
Macular pigment assessment modalities encompass various techniques to measure the concentration and distribution of macular pigment in the eye, serum, and skin. While several approaches exist for macular pigment measurements, these well-established, validated modalities that are practical and most commonly used in research settings hold the potential to contribute to a comprehensive understanding of macular pigment status in the eye, skin, and serum, aiding in preventive eye care and personalized interventions for individuals at risk of degenerative eye conditions such as AMD.
Macular Pigment Measurements Most Commonly Used In Research Settings
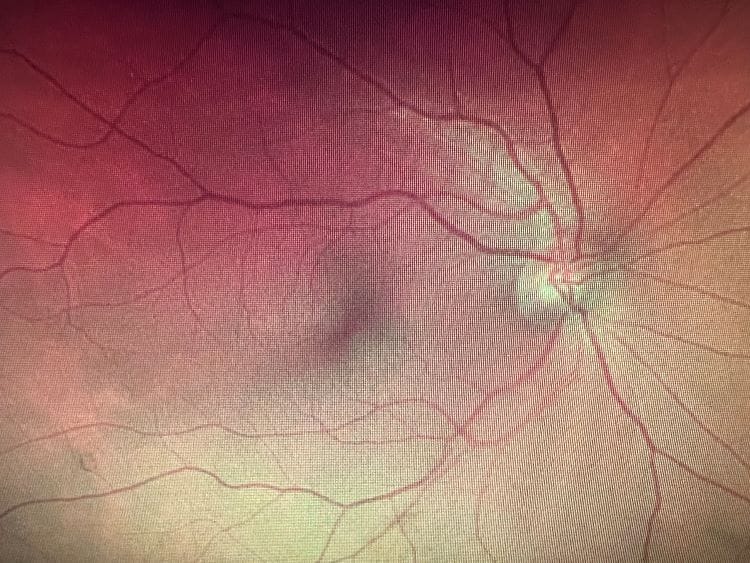
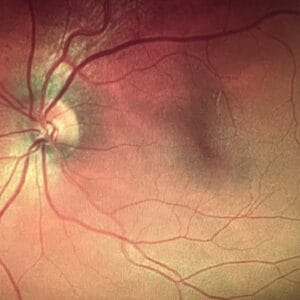
Retinal Measurements: Macular pigment can be measured directly using physical (i.e., autofluorescence) and psychophysical (i.e., customized heterochromatic flicker photometry) imaging techniques.1, 11 The dual-wavelength autofluorescence imaging (AFI) takes advantage of the differential absorption of light by macular pigment and fluorophores (primarily lipofuscin, a substance that accumulates in the retinal pigment epithelium with age).12, 13 This technique uses two different wavelengths of light, typically short (blue) and long (green) wavelengths. The macular pigment absorbs more of the short-wavelength light, causing a reduction in the autofluorescence signal produced by lipofuscin at the blue wavelength. The difference between the autofluorescence signals at these two wavelengths indicates the macular pigment optical density. AFI offers spatial mapping at a relatively high resolution, providing detailed insights into the distribution of macular pigment across the retina.14
The customized heterochromatic flicker photometry (cHFP) technique relies on the differential absorption of light by macular pigment.15, 16 The cHFP aims to determine the density of macular pigment in the central retina. In cHFP, the individual is presented with flickering lights of different colors, typically blue and green, and the task is to detect a point where the flicker appears to cease or minimize. The wavelength at which this flicker fusion occurs is related to macular pigment density. The absorption characteristics of macular pigment cause a reduction in the perceived flicker, and this point of equilibrium provides a measure of macular pigment optical density. The cHFP provides a spatial profile of macular pigment density across the macula at a limited number of eccentricities. This spatial mapping allows for assessing macular pigment level variations at different central retina locations. Researchers and clinicians use cHFP to understand how macular pigment density correlates with visual function and to investigate its potential role in conditions such as AMD.
While AFI and cHFP are valuable tools, it is essential to note that their assessment could be influenced by factors such as the participant’s age, lens density, and other ocular conditions. Despite these considerations, AFI and cHFP remain widely used and scientifically validated methods for measuring macular pigment levels, contributing to our understanding of ocular health and diseases.11, 13
Skin Measurements: Resonance Raman spectroscopy (RRS) and pressure-mediated reflectance spectroscopy (RS) devices are techniques used to measure skin carotenoid levels, offering an understanding of an individual’s fruit and vegetable intake.17, 18 These techniques are non-invasive, validated, and rapid methods of measuring carotenoid status in skin as a biomarker of fruit and vegetable intake.19-22 RRS operates on the principle of Raman scattering, where the skin (the palm) is exposed to monochromatic light, often in the blue-green range, resonant with the absorption bands of carotenoids. The scattered light is then analyzed, and the intensity of the Raman signal provides a quantitative measure of skin carotenoid levels. RRS is highly specific to the molecular structure of carotenoids, offering accurate and precise measurements of an individual’s nutritional status based on skin carotenoid concentrations. It is worth noting that RRS systems are custom built and available only for research purposes.
Pressure-mediated reflectance spectroscopy with the commercially available Veggie Meter uses gentle pressure on the index finger, which momentarily squeezes blood out of the illuminated tissue volume, reducing oxyhemoglobin and other chromophores’ influence on the reflection spectra.23 The Veggie Meter is a validated and portable device that measures the skin carotenoid score and is significantly correlated with skin RRS, serum total carotenoids, and macular pigment.24-27 The device illuminates the index finger with broad-band white light, analyzes diffusively reflected light in real time, and provides a skin carotenoid score on a scale from 0 to 800 within ~10 seconds. This practical technique offers a quick assessment of an individual’s carotenoid status, especially in settings where a more accessible and user-friendly approach is required.
RRS and RS are important for assessing skin carotenoids, reflecting an individual’s dietary intake of carotenoid-rich foods. RRS provides high specificity and precision, while RS offers practicality and ease of use, making them suitable for various research and clinical applications. Together, these techniques contribute to a comprehensive understanding of an individual’s carotenoid status, with potential implications for overall health and well-being.
Serum Measurements: Serum carotenoid analysis using high-performance liquid chromatography (HPLC) is a widely employed technique in nutritional and clinical research to quantify and identify various carotenoids in the blood.28, 29 As a gold standard in carotenoid analysis, HPLC provides accurate and reproducible results, contributing significantly to our understanding of the role of carotenoids in human health and disease prevention. The process involves extracting carotenoids from the serum and chromatographic separation using a high-pressure liquid mobile phase. The separated compounds are then detected and quantified based on their characteristic absorption spectra. HPLC is favored for its high sensitivity, precision, and ability to separate individual carotenoid compounds from complex mixtures in serum samples. While HPLC is valuable in research, its application in routine ophthalmic clinic settings for preventive care may be limited due to cost, time, and the need for specialized equipment.
Benefits of Routine Macular Pigment Measurements
Macular pigment assessment may play a crucial role in the risk assessment for AMD. Here are the potential benefits of incorporating such assessments into routine eye health screenings.
Early Detection and Intervention: Macular pigment acts as a natural filter, protecting the retina from harmful high-energy light.1 Low macular pigment density is associated with a higher risk of AMD, a leading cause of vision loss in older adults.30, 31 Hence, macular pigment assessment can promote the early detection of suboptimal levels, allowing for timely interventions such as dietary changes or supplementation to enhance macular health.32 By measuring macular pigment, clinicians can identify changes associated with AMD risk before significant vision loss occurs.
Personalized Nutrition Guidance: Individual variations in macular pigment levels necessitate personalized nutritional recommendations. Macular pigment measurements guide the development of tailored dietary plans to support ocular health. Individuals with lower macular pigment may be advised to incorporate specific dietary changes or nutritional supplements rich in lutein and zeaxanthin, known to support macular health and potentially reduce the risk of AMD progression.32
Visual Performance Optimization: Adequate macular pigment is linked to improved visual performance. Thus, optimizing macular pigment levels improves contrast sensitivity, reduces glare sensitivity, and improves adaptation to varying light conditions.9, 10 This proactive approach not only aids in preserving overall visual acuity but also enhances visual comfort and quality, making routine macular pigment assessment essential for promoting and maintaining optimal visual performance.
In conclusion, macular pigment measurements are integral to modern clinical examinations, offering a window into an individual’s ocular health. Continued research into the relationship between macular pigment and ocular health will further enhance our understanding and refine clinical applications. The combination of precise assessment modalities and personalized interventions holds great promise for preventing and managing eye conditions such as AMD.
References
1 Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, Nolan JM. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Progress in retinal and eye research 2016;50:34-66.
2 Bone RA, Landrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision research 1985;25:1531-1535.
3 Perry A, Rasmussen H, Johnson EJ. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. Journal of Food Composition and Analysis 2009;22:9-15.
4 Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annual review of nutrition 2003;23:171-201.
5 Li B, Ahmed F, Bernstein PS. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Archives of biochemistry and biophysics 2010;504:56-60.
6 Seddon JM. Macular Degeneration Epidemiology: Nature-Nurture, Lifestyle Factors, Genetic Risk, and Gene-Environment Interactions – The Weisenfeld Award Lecture. Investigative ophthalmology & visual science 2017;58:6513-6528.
7 Johnson EJ. A possible role for lutein and zeaxanthin in cognitive function in the elderly. The American journal of clinical nutrition 2012;96:1161S-1165S.
8 Stringham JM, Johnson EJ, Hammond BR. Lutein across the Lifespan: From Childhood Cognitive Performance to the Aging Eye and Brain. Curr Dev Nutr 2019;3:nzz066.
9 Nolan JM, Power R, Stringham J, et al. Enrichment of Macular Pigment Enhances Contrast Sensitivity in Subjects Free of Retinal Disease: Central Retinal Enrichment Supplementation Trials – Report 1. Investigative ophthalmology & visual science 2016;57:3429-3439.
10 Stringham JM, O’Brien KJ, Stringham NT. Macular carotenoid supplementation improves disability glare performance and dynamics of photostress recovery. Eye and vision (London, England) 2016;3:30.
11 Akuffo KO, Beatty S, Stack J, et al. Concordance of Macular Pigment Measurement Using Customized Heterochromatic Flicker Photometry and Fundus Autofluorescence in Age-Related Macular Degeneration. Investigative ophthalmology & visual science 2015;56:8207-8214.
12 Addo EK, Gorusupudi A, Allman S, Bernstein PS. The Lutein and Zeaxanthin in Pregnancy (L-ZIP) study-carotenoid supplementation during pregnancy: ocular and systemic effects-study protocol for a randomized controlled trial. Trials 2021;22:300.
13 Green-Gomez M, Bernstein PS, Curcio CA, Moran R, Roche W, Nolan JM. Standardizing the Assessment of Macular Pigment Using a Dual-Wavelength Autofluorescence Technique. Translational Vision Science & Technology 2019;8:41-41.
14 You QS, Bartsch DU, Espina M, Alam M, Camacho N, Mendoza N, Freeman WR. Reproducibility of Macular Pigment Optical Density Measurement by Two-Wavelength Autofluorescence in a Clinical Setting. Retina (Philadelphia, Pa) 2016;36:1381-1387.
15 Dennison JL, Stack J, Beatty S, Nolan JM. Concordance of macular pigment measurements obtained using customized heterochromatic flicker photometry, dual-wavelength autofluorescence, and single-wavelength reflectance. Experimental eye research 2013;116:190-198.
16 Wooten BR, Hammond BR, Jr., Land RI, Snodderly DM. A practical method for measuring macular pigment optical density. Investigative ophthalmology & visual science 1999;40:2481-2489.
17 Mayne ST, Cartmel B, Scarmo S, Jahns L, Ermakov IV, Gellermann W. Resonance Raman spectroscopic evaluation of skin carotenoids as a biomarker of carotenoid status for human studies. Archives of biochemistry and biophysics 2013;539:163-170.
18 Ermakov IV, Whigham LD, Redelfs AH, Jahns L, Stookey J, Bernstein PS, Gellermann W. Skin Carotenoids as Biomarker for Vegetable and Fruit Intake: Validation of the Reflection-Spectroscopy Based “Veggie Meter”. The FASEB Journal 2016;30 409.403-409.403.
19 Jahns L, Johnson LK, Conrad Z, et al. Concurrent validity of skin carotenoid status as a concentration biomarker of vegetable and fruit intake compared to multiple 24-h recalls and plasma carotenoid concentrations across one year: a cohort study. Nutrition journal 2019;18:78.
20 Jahns L, Johnson LK, Mayne ST, et al. Skin and plasma carotenoid response to a provided intervention diet high in vegetables and fruit: uptake and depletion kinetics. The American journal of clinical nutrition 2014;100:930-937.
21 Mayne ST, Cartmel B, Scarmo S, et al. Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. The American journal of clinical nutrition 2010;92:794-800.
22 Scarmo S, Henebery K, Peracchio H, et al. Skin carotenoid status measured by resonance Raman spectroscopy as a biomarker of fruit and vegetable intake in preschool children. European journal of clinical nutrition 2012;66:555-560.
23 Ermakov IV, Gellermann W. Dermal carotenoid measurements via pressure mediated reflection spectroscopy. Journal of biophotonics 2012;5:559-570.
24 Conrady CD, Bell JP, Besch BM, et al. Correlations Between Macular, Skin, and Serum Carotenoids. Investigative ophthalmology & visual science 2017;58:3616-3627.
25 Ermakov IV, Ermakova M, Sharifzadeh M, et al. Optical assessment of skin carotenoid status as a biomarker of vegetable and fruit intake. Archives of biochemistry and biophysics 2018;646:46-54.
26 Henriksen BS, Chan G, Hoffman RO, Sharifzadeh M, Ermakov IV, Gellermann W, Bernstein PS. Interrelationships between maternal carotenoid status and newborn infant macular pigment optical density and carotenoid status. Investigative ophthalmology & visual science 2013;54:5568-5578.
27 Thapa R, Addo EK, Ruit S, Bernstein PS. Assessment of Skin Carotenoid Measurement as a Means to Detect Vitamin A Deficiency, in Children and Pregnant Women of Nepal. The Journal of nutrition 2023.
28 Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Investigative ophthalmology & visual science 1988;29:843-849.
29 Li B, Gorusupudi A, Arunkumar R, Bernstein PS. Chapter Six – Extraction, detection, and imaging of the macular carotenoids. In: Wurtzel ET (ed), Methods in Enzymology: Academic Press; 2022:185-213.
30 Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. Jama 1994;272:1413-1420.
31 Mares-Perlman JA, Fisher AI, Klein R, Palta M, Block G, Millen AE, Wright JD. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. American journal of epidemiology 2001;153:424-432.
32 Chew EY, Clemons TE, Agrón E, et al. Long-term Outcomes of Adding Lutein/Zeaxanthin and ω-3 Fatty Acids to the AREDS Supplements on Age-Related Macular Degeneration Progression: AREDS2 Report 28. JAMA ophthalmology 2022;140:692-698.