April 11, 2023
The introduction of the anti-VEGF drugs beginning in 2006 for the treatment of neovascular AMD (nAMD), followed a few years later with their approval for diabetic macular edema (DME), revolutionized the management for these conditions. Prior to these medications, laser photocoagulation and photodynamic therapy (PDT) for AMD were considered the standard of care, and indeed they “kind of” worked, but the reality was that at the end of the day, patients often continued to lose vision over time.
Consider that for AMD patients with subfoveal choroidal neovascularization (CNV), there was a clinical trial funded by the National Eye Institute comparing laser photocoagulation of the fovea vs. observation, with the hypothesis that the scar that would develop from lasering the fovea would be smaller than the scar that would develop from the natural course of the disease. Never mind that the patient whose fovea got lasered automatically dropped to 20/200 or worse, in most cases.
In patients with clinically significant macular edema, the goal of laser was not to improve vision but rather to keep patients with the same visual acuity level as when they started. So, if a patient presented with 20/80 acuity, the goal of laser was not to improve vision but to keep them at 20/80. Often patients did improve a little, but that was not the goal.
PDT for nAMD was a little better, but once again the visual outcomes still resulted in a slow progressive worsening of vision over time. What’s more, both laser and PDT required well defined CNV on fluorescein angiography, which unfortunately limited access and success as most patients presented with poorly defined or occult CNV. Or, they presented with CNV involving the fovea, which further limited the success.
Anti-VEGF Drugs Were a Game Changer
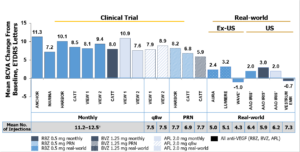
Indeed, the anti-VEGF drugs were a game changer! Though pegaptanub (Macugen) was the first FDA-approved anti-VEGF drug for treating nAMD, it wasn’t until ranibizumab (Lucentis) and bevacizumab (Avastin), followed by aflibercept (Eylea), that for the first time ever we were actually seeing patients having a significant improvement in visual acuity. In the pivotal AMD trials, patients treated with an anti-VEGF medication on average had a 9- to 11-letter visual acuity gain over two years compared to the usual care in which patients lost on average almost 10 letters of acuity.1-5 In the diabetic macular edema (DME) trials, patients on average gained approximately 12 letters of acuity vs. the usual care of only 2.5 letters over a two-year period.6-7
As good as the clinical trial results were for both AMD and DME, the reality is the treatment burden for patients with these conditions was exhaustive. Many patients required frequent injections – often monthly, to sustain the levels of improved vision. In fact, “real world” visual acuity results were not nearly as good as what was seen in the clinical trials. In the American Academy of Optometry IRIS registry, at one year, AMD patients treated for CNV had only a two-letter improvement in their vision, and the diabetic macular edema outcomes were not much better where DME patients at one year had only a four-letter gain.8-9 What’s more, fewer than 50% of patients receiving anti-VEGF therapy for DME received less than three injections over the first year.10 This highlights not only the poor durability of the current anti-VEGF drugs but also the treatment burnout that patients experience with needing frequent injections.
The Next Generation
Fortunately, newer and more durable medications have emerged that should result in better visual outcomes as well as reducing the treatment burden on both patients and retinal specialists.
Brolucizumab (Beovu) was FDA approved in 2019 with much hope and fanfare with the expectation that it would work equally well as the current medications but have more durability resulting in less frequent dosing. Indeed, the pivotal FDA clinical trials showed exactly that with over half the patients being maintained on every 12-week dosing, or approximately four injections per year, after the initial loading dose.11 It also seemed to do a better job of getting rid of intraretinal and/or subretinal fluid than ranibizumab, the control. Unfortunately, once brolucizumab started to be used in clinical practice, several cases were reported of severe inflammation following injection resulting in near-blinding retinal vasculitis and vascular occlusions;12-13 thus essentially putting an end to retinal specialists using brolucizumab.
Fortunately, in January 2022, faricimab was approved to treat both nAMD and DME with similar expectations of better durability and less frequent injections than the current medications. Faricimab is the first bispecific antibody that works to inhibit VEGF-A in addition to the angiopoietin-2 pathway, a critical driver for angiogenesis and leakage as well as microvascular inflammation. In separate clinical trials studying the treatment affect in nAMD and DME, patients were randomized to every 12 weeks or every 16 weeks compared to monthly injections for nAMD or every eighth week injections of ranibizumab in DME.
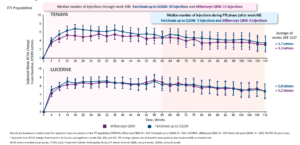
In both nAMD and DME, dosing every 12 weeks and every 16 weeks had similar visual acuity outcomes with both groups achieving over a 10-letter improvement at one year compared to the ranibizumab treated eyes. In fact, in the DME trials, over 80% of patients treated with faricimab were able to maintain every 12- or 16-week dosing through 100 weeks.14-15 What’s more, the safety signals were very good with no reported cases of inflammation or retinal vasculitis.
So far, faricimab has lived up to the expectations of better durability without the burden of every month or every other month injection. Unfortunately, there are some hurdles that retinal specialists need to jump through to be able to use this drug. Most insurance payers require a prior authorization and/or step therapy for approving faricimab. This includes documenting an inadequate treatment response, intolerance, or contraindication to bevacizumab. Patients with DME must have a baseline visual acuity of 20/50 or worse and must have an inadequate treatment response to bevacizumab or ranibizumab. Hopefully, with time, payers will see the wisdom and allow better access because these newer treatments will ultimately save money by reducing the number of treatments as well as the number of visits to the retinal specialist.
The port delivery system (PDS) is another breakthrough treatment modality that offers extended-release dosing of ranibizumab with the goal of potentially never needing an intravitreal injection. The PDS is a permanent refillable device that gets implanted through the sclera and works by continuously delivering ranibizumab into the vitreous cavity for up to six months. The port is surgically implanted in an operating room via an out-patient procedure. It is refilled with unique syringe into a self-sealing septum that removes any residual medication while also refilling the reservoir with a “refill-exchange procedure.” The clinical trial results showed the PDS was just as good as monthly injections of ranibizumab. Patients in the injection arm of the study averaged around 10 injections over 40 weeks. Only four of 228 patients in the PDS arm at six months required refilling the device16-17.
Unfortunately, in October 2022, Genentech did a voluntary recall on the PDS due to dislodgement of the septum.18 This is likened to a wine cork being pushed down into the wine bottle. Unfortunately, this prevents being able to safely refill the reservoir. The company is working to re-engineer the device to prevent this from recurring. Patients who have already received the PDS can continue refill exchange procedures.
This Is Just the Beginning of What It Could Mean for Optometry
For optometry, this could mean more opportunities for co-management, or at the very least being able to follow our patients in the interim between visits with the retinal specialist. For those with OCTs it may be another opportunity to scan the macula to ensure patients are getting better. Finally, as the retinal anatomy improves with more sustainable treatments, we may also see patients coming in to update their spectacle correction sooner than before as the visual function improves more quickly.
 All of these are opportunities to keep patients connected to our practices while also generating revenue.
All of these are opportunities to keep patients connected to our practices while also generating revenue.
The first generation of any new treatment is an important first step but is often short-lived as advances in technology usually pave the way for what’s to come next. The release of the anti-VEGF medications 17 years ago was an important first step, and though it took a while, we are now witnessing the evolution of the next generation of treatments that work just as well as the original medication but are more durable and seem to reduce the treatment burden on our patients, while hopefully allowing them to enjoy better vision for their lifetime.
Though we have seen incredible advances in the treatment for retinal diseases, the final chapter by no means has been written. One only needs visit ClinicalTrials.gov to see the massive numbers of clinical trials that are currently underway. Certainly, there is a lot focusing on treatments for dry and wet AMD as well as diabetic retinopathy; particularly new drugs as well as better delivery methods. There is even a promising study by OcuTerra Therapeutics utilizing an eye drop for treating diabetic retinopathy. There is also a focus around genetics, not just for treating hereditary retinal conditions but even around AMD, using adenoviral vectors to deliver genetic material to modify or change gene expression. There are also gene therapy studies targeting the complement pathway. Though many of these studies are in the early phases, I think we are witnessing the dawn of a new age in treating eye disease, and what we have seen so far is just the beginning.
References
1 Rosenfeld PJ, Brownb DM, Haier JS et al. Ranibizumab for Neovascular Age-Related Macular Degeneration. N Engl J Med 2006; 355:1419-1431
2 Brown DM, Kaiser PK, Michaels M, et al. Ranibizumab versus Verteporfin for Neovascular Macular Degeneration. Brown DM, et al. N Engl J Med. 2006;355:1432-1444.
3 Ranibizumab and Bevacizumab for Neovascular Age-Related Macular Degeneration. CATT Research Study Group. N Engl J Med. 2011;364(20):1897-1908
4 Nguyen QD, Heier J, Brown D, et al. Randomized, double-masked, active-controlled phase 3 trial of the efficacy and safety of intravitreal VEGF trap-eye in wet AMD: one-year results of the View-1 study Invest Ophthalmol Vis Sci. 2011;52:E-Abstract 3073.
5 Schmidt-Erfurth U, Chong V, Kirchof B, et al. Primary results of an international phase III study using intravitreal VEGF trap-eye compared to ranibizumab in patients with wet AMD (VIEW 2). Invest Ophthalmol Vis Sci. 2011;52:E-Abstract 1650
6 Brown DM, Nguyen DQ, Marcus, DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013-2022.
7 Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema, Ophthalmology. 2014;121:2247-2254.
8 Cantrell RA, Lum F, Yifeng, et al. Treatment Patterns for Diabetic Macular Edema: An Intelligent Research in Sight (IRIS®) Registry Analysis. Ophthalmology 2020; 127(3):427-429
9 Rao P, Lum F, Wood K, et al. Real-World Vision in Age-Related Macular Degeneration Patients Treated with Single Anti–VEGF Drug Type for 1 Year in the IRIS Registry. Ophthalmology. 2018;125(4):522-528.
10 Ciulla, TA, Huang F, Westby K, et al. Real-world Outcomes of Anti-Vascular Endothelial Growth Factor Therapy in Neovascular Age-Related Macular Degeneration in the United States. Ophthalmology Retina. 2018 Jul;2(7):645-653.
11 Dugel, PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. 2020; 127: 72-84.
12 Haug SJ, Hien DL, Uludag G, et al. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am J Ophthalmol Case Rep. 2020;18
13 Jain A, Chea S, Matsumiya W, et al. Severe vision loss secondary to retinal arteriolar occlusions after multiple intravitreal brolucizumab administrations. Am J Ophthalmol Case Rep. 2020;18:
14 Heier JS, Khanani KM, Ruiz CQ et al. Efficacy, Durability, and Safety of Intravitreal Faricimab up to Every 16 Weeks for Neovascular Age-Related Macular Degeneration (TENAYA and LUCERNE): Two Randomised, Double-Masked, Phase 3, Non-Inferiority Trials. Lancet 2022; 399: 729–40.
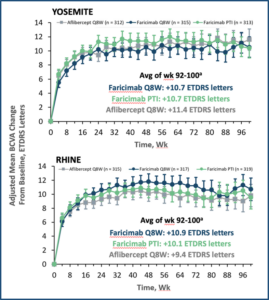
15 Wykoff CC, Abreu F, Adamis AP, et al. Efficacy, Durability, and Safety of Intravitreal Faricimab with Extended Dosing up to Every 16 Weeks in Patients with Diabetic Macular Oedema (YOSEMITE and RHINE) two randomised, double-masked, phase 3 trials. Lancet 2022; 399: 741-755.
16 Campochiaro PA, Marcus DM, Awh CC, et al. The Port Delivery System with ranibizumab for neovascular age-related macular degeneration: results from the randomized phase 2 ladder clinical trial. Ophthalmology 2019; 126:1141–54.
17 Holekamp NM, Campochiaro PA, Chang M, et al. Archway randomized phase 3 trial of the Port Delivery System with ranibizumab for neovascular age-related macular degeneration. Ophthalmology 2022; 129:295–307.
18 Voluntary recall of the SUSVIMOTM Ocular Implant https://www.gene.com/download/pdf/Susvimo_DHCP_Important_Prescribing_Information_2022-10-18.pdf