September 12, 2023
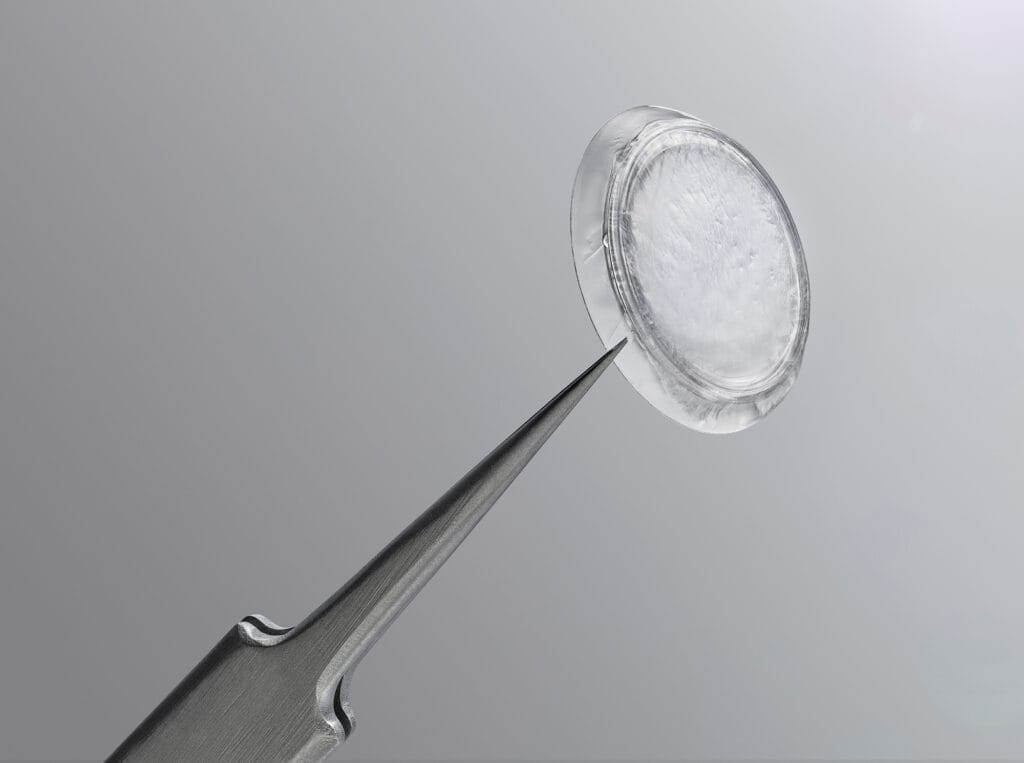
Since ocular surface disease is more prevalent as we age, it’s important to understand the different therapeutic options to treat this condition. One highly effective treatment is the use of amniotic membranes. Amniotic membranes are the substrate to culture and deliver limbal epithelial stem cells to the ocular surface.1 An amniotic membrane is obtained from placentas donated by individuals who undergo cesarean section. It is a rich source of biologically active elements that can facilitate the process of healing.2 These membranes promote epithelialization and demonstrate anti-inflammatory, anti-fibrotic, anti-angiogenic, and anti-microbial features.2
The preparation of an amniotic membrane is a meticulous process. After serological screening of maternal blood six months postpartum to rule out the potential transmission of communicable diseases, amniotic membrane is bluntly dissected, then usually transferred onto a nitrocellulose paper carrier, typically with the epithelial side up, and cut into multiple pieces of various dimensions.2 Although these membranes are an incredible treatment tool, it should be noted that obtaining amniotic membrane can be a costly process as it requires rigorous donor screening, specialized manipulation techniques, and securing the substrate can be challenging.3
Treatment Used More Than Ever Before
Amniotic membranes in eye care are used more than ever before for ocular surface reconstruction, including the treatment of persistent epithelial defects and non-healing corneal ulcers, corneal perforations and descemetoceles, bullous keratopathy, limbal stem cell deficiency, pterygium, conjunctival reconstruction, corneoscleral melts and perforations, and glaucoma surgery.2
The utilization of amniotic membranes is expanding beyond eye care to other specialties including dermatology, plastic surgery, genitourinary medicine, and otolaryngology. Amniotic membrane have an extensive use in regenerative medicine and potentially anticancer treatment.2
Types of Amniotic Membranes
There are two types of amniotic membranes, cryopreserved or dehydrated tissue.
Cryopreserved:
The only FDA-cleared cryopreserved amniotic membrane is Prokera (BioTissue), which sustains the corneal healing process without harmful side effects.4 The CryoTek preservation method supports biologic components including a key protein component HC-HA/PTX3, and structural integrity alike fresh tissue, which aids in rapidly restoring the cornea’s own healing capabilities.5,6
Multiple Prokera products are available. Prokera Classic maintains the orbital space with a symblepharon ring when cases of prevention of closure or adhesion are a concern. Prokera Slim is a lower profile device that contours to the ocular surface to maintain comfort using ComfortRing technology. Prokera Plus is used for patients who need intensive treatment and maximizes the therapeutic benefit with a double layer of cryopreserved amniotic membrane. Prokera Clear has a 6mm ClearView aperture to provide visual acuity during treatment for monocular patients.
To maintain their viability, cryopreserved amniotic membranes must be kept frozen until they are ready for use. These membranes are typically stored in an antibiotic and antifungal solution, which makes them potentially problematic for allergic patients and necessitates their careful use.
Dehydrated:
There are a variety of dehydrated membranes on the market such as BioDOptix, Integra LifeSciences; AmbioDisk, IOP Ophthalmics; Opticyte, Merakris; Aril, Seed Biotech; and Apollo, Atlas Ocular. A specialized dehydration process is used to create dehydrated amniotic membranes, whereby the water content of the natural amniotic membrane is extracted. This process yields a thin, flexible, and translucent membrane that still maintains the natural extracellular matrix, growth factors, and cytokines present in the original tissue.7 Dried membranes can be stored at room temperature for up to five years, and they are comparatively less expensive than cryopreserved amniotic membranes. After placement on the eye, a bandage contact lens is typically applied over the amniotic membrane to secure it in position. Like the cryopreserved membrane, it can dissolve over the course of two to six days depending on the level of underlying inflammation.
Amniotic membranes offer a promising therapeutic option for ocular surface disease, with anti-inflammatory, antimicrobial, and wound-healing properties. They offer a safe, effective, and minimally invasive approach to treating a range of ocular surface conditions, making them a valuable treatment option for our ocular surface disease patients.
References
1 Levis HJ, Kureshi AK, Massie I, Morgan L, Vernon AJ, Daniels JT. Tissue Engineering the Cornea: The Evolution of RAFT. J Funct Biomater. 2015 Jan 22;6(1):50-65. doi: 10.3390/jfb6010050. PMID: 25809689; PMCID: PMC4384100.
2 Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting-a review. Cell Tissue Bank. 2017 Jun;18(2):193-204. doi: 10.1007/s10561-017-9618-5. Epub 2017 Mar 2. PMID: 28255771.
3 Bobba S, Di Girolamo N. Contact Lenses: A Delivery Device for Stem Cells to Treat Corneal Blindness. Optom Vis Sci. 2016 Apr;93(4):412-8. doi: 10.1097/OPX.0000000000000699. PMID: 26390346.
4 Thomasen H, Pauklin M, Steuhl KP, Meller D. Comparison of cryopreserved and air-dried human amniotic membrane for ophthalmologic applications. Graefes Arch Clin Exp Ophthalmol. 2009 Dec;247(12):1691-700. doi: 10.1007/s00417-009-1162-y. Epub 2009 Aug 20. PMID: 19693529.
5 Tan EK, Cooke M, Mandrycky C, et al. Structural and biological comparison of cryopreserved and fresh amniotic membrane tissues. J Biomater Tissue Eng. 2014;(4):379–388.
6 Cooke M, Tan EK, Mandrycky C, et al. Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue.
7 Cooke M, Tan EK, Mandrycky C, He H, O’Connell J, Tseng SC. Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue. J Wound Care. 2014 Oct;23(10):465-74, 476. doi: 10.12968/jowc.2014.23.10.465. PMID: 25296347.
Arrizabalaga JH, Nollert MU. Human Amniotic Membrane: A Versatile Scaffold for Tissue Engineering. ACS Biomater Sci Eng. 2018 Jul 9;4(7):2226-2236. doi: 10.1021/acsbiomaterials.8b00015. Epub 2018 May 22. PMID: 33435098.