February 23, 2024
New developments in cancer therapy include treatment with antibody-drug conjugates (ADCs), which are a targeted cancer therapy. Platinum-based chemotherapy is the primary treatment for patients with epithelial ovarian, fallopian, or primary peritoneal cancer. This treatment is initially effective, but most patients eventually relapse, developing platinum-resistant cancer. Treatment options at this stage have been limited, and the ones available have poor response rates, short duration of response, and considerable toxicity and tolerability limitations.1
ADCs are comprised of a tumor-specific monoclonal antibody with a potent anti-cancer agent. The monoclonal antibody is directed to specific cell surface targets (e.g., ovarian cancer), and the entire conjugate is endocytosed into the cell bringing with it the cytotoxin (anti-cancer agent). Once inside the cell, the cytotoxin is released from the antibody, resulting in cancer cell death.2 Mirvetuximab soravtansine (MIRV) is a first-in-class ADC indicated for the treatment of adult patients with FRα-positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer who have received one to three prior systemic treatment regimens.1
Targeted Cancer Treatment Linked to Ocular Adverse Events
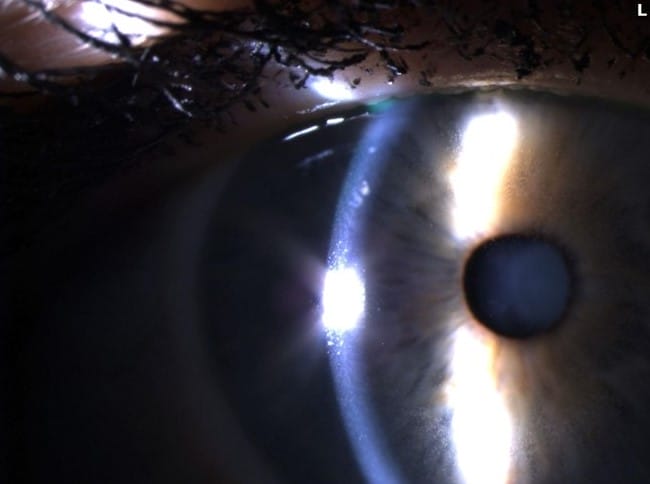
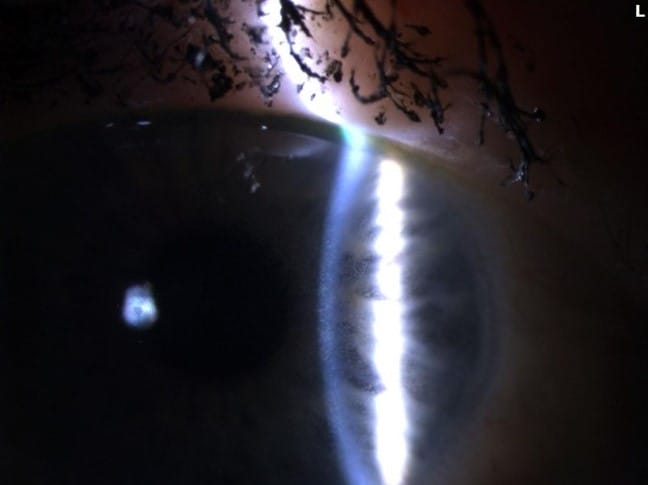
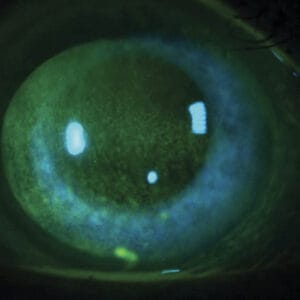
The ADCs have known ocular adverse events (OAEs). Various side effects have been reported relating to the corneal deposition of these drugs, including: microcyst-like epithelial changes, intraepithelial spherical lesions, whorl-like keratopathy, corneal epitheliopathy, corneal changes, blurred vision, and keratitis.3 The underlying mechanisms of ocular toxicities remain poorly understood, but it is hypothesized to be an off-target effect on the corneal epithelium due to the lack of FRα- receptors in that part of the eye.4 With MIRV treatment, the most common OAEs are blurred vision likely secondary to the development of keratopathy.5 The most common ocular sign of OAEs is the development of epithelial microcysts and keratitis, which can lead to corneal haze and decreased vision (see Figures 1-3).
Figures 1-3. Epithelial microcysts and keratitis can result in corneal haze and decreased vision
Blurred vision, keratitis, dry eye, and photophobia are the most common OAEs that are assessed to monitor the effects of MIRV treatment. The patient should be evaluated prior to the initiation of treatment and then during treatment. Blurred vision and keratitis are assessed on a scale of 1-4, while dry eye and photophobia are graded on a scale of 1-3.6 The onset of OAEs typically occurs during the second cycle of treatment (blurred vision ≈ 1.3 months, keratopathy ≈ 1.5 months).9 The OAE profile of MIRV is a dose-dependent toxicity limited to the corneal epithelium, which is resolvable with treatment and alteration to the treatment dosage of MIRV.1 At grading scale of 3, patients are symptomatic with marked decrease in visual acuity that affects daily living (ADL). At this stage, referral back to their oncologist is recommended to determine if their MIRV treatment needs to be adjusted.6 Less than 1% of patients had to discontinue MIRV treatment secondary to OAEs,10 and 57% of patients with OAEs did not require a dose delay or reduction.9
Prior to the initiation of MIRV therapy, an ocular assessment is required and then every second cycle (each cycle of treatment is three weeks, so every six weeks) for the first six months of treatment. A detailed anterior segment exam and refraction are necessary at the initial assessment to monitor for changes during treatment. The Ocular Assessment form is a useful tool to be used for the examination of these patients. You should also review these important billing and coding details.
MIRV Study Results and Recommendations
The clinical trials regarding MIRV demonstrated that pre-treatment eye drops, ophthalmic monitoring, and dose modifications by the patient’s oncologist are crucial for the best treatment outcomes for the patient (See eye care guidelines table). Prophylactic use of steroid drops and frequent lubrication with preservative-free artificial tears has been shown to reduce the OAEs with MIRV treatment. Careful examination and documentation of any shifts in refractive error or corneal changes are not only important for the managing of these OAEs, but they play an important role in guiding the oncologist on potentially altering the MIRV dosage (to delay, reduce, or permanently discontinue treatment based on severity and persistence of symptoms).8
The recommended MIRV dose change is based on the patient’s OAEs. The goal is to enable prompt intervention with dosage modification by the oncologist to limit unnecessary discontinuations in treatment.5 It is important to note that treatment at the recommended dose of MIRV is preferable. However, it was found that for those patients who required a dose reduction, it did not appear to affect treatment outcomes.9
Clinical Pearl: Optometrists play a crucial role in the management of patients undergoing MIRV therapy. OAEs are a common finding with MIRV therapy, and the optometrist’s role is to not only diagnose/manage any corneal/refractive changes but to report those to the oncologist for potential MIRV dose modification. I think optometrists may feel conflicted in reporting any or the severity of OAEs in fear that the patient may have their treatment discontinued. The presence and severity of OAEs is crucial for the oncologist to potentially modify the MIRV dosage to allow the patient to continue the therapy. Even though the treatment of MIRV at the recommended dose is preferred, a reduction in dose does not appear to result in suboptimal treatment. Close monitoring for OAEs is not only crucial so that they can be managed by the optometrist, but because they are important indicators to the oncologist to modify the dose so that the patient is able to stay on treatment.
References
1 Kuroki, L., Guntupalli, S.R., 2020. Treatment of epithelial ovarian cancer. BMJ 371, m3773.
2 Canestraro J, Hultcrantz M, Modi S, Hamlin PA, Shoushtari AN, Konner JA, Tew WP, Iyengar NM, Heinemann M, Abramson DH, Francis JH. Refractive Shifts and Changes in Corneal Curvature Associated With Antibody-Drug Conjugates. Cornea. 2022 Jun 1;41(6):792-801. doi: 10.1097/ICO.0000000000002934. Epub 2021 Nov 23. PMID: 34839332; PMCID: PMC9106803.
3 Eaton JS, Miller PE, Mannis MJ et al. Ocular adverse events associated with anti-body drug conjugates in human clinical trials. J Ocul Pharmacol Ther 2015;21(10):589–604.
4 Matulonis UA, Birrer MJ, O’Malley DM, Moore KN, Konner J, Gilbert L, Martin LP, Bauer TM, Oza AM, Malek K, Pinkas J, Kim SK. Evaluation of Prophylactic Corticosteroid Eye Drop Use in the Management of Corneal Abnormalities Induced by the Antibody-Drug Conjugate Mirvetuximab Soravtansine. Clin Cancer Res. 2019 Mar 15;25(6):1727-1736. doi: 10.1158/1078-0432.CCR-18-2474. Epub 2018 Nov 9. PMID: 30413525.
5 ELAHERE Prescribing Information. ImmunoGen. 2022. Accessed February 7, 2024.
6 National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 5. US Department of Health and Human Services; 2017. Published November 27, 2017. Accessed February 7, 2024.
7 Hendershot A, Slabaugh M, Riaz KM, et al. Strategies for prevention and management of ocular events occurring with mirvetuximab soravtansine. Gynecol Oncol Rep. 2023;47:101155.
8 Connolly, Sarah and Lytle, Grace. Managing Microcyst-Like Epithelial Keratopathy Related to Cancer Therapy: Facts optometrists need to know. Modern Optometry, October 2023.
9 Matulonis, U.A., Oaknin, A., Pignata, S., Denys, H., Colombo, N., Van Gorp, T., et al., 2022. Mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: characterization of anti-tumor activity in the SORAYA study. Poster presented at: 2022 American Society of Clinical Oncology Annual Meeting; June 3-7, 2022; Chicago, IL.
10 Moore, K.N., Lorusso, D., Oaknin, A., Oza, A., Colombo, N., Van Gorp, T., et al., 2022. Integrated safety summary of single-agent mirvetuximab soravtansine in patients with folate receptor alpha (FRα)-positive recurrent ovarian cancer: Phase 1 and 3 clinical trials. Presented at: 2022 American Society of Clinical Oncology Annual Meeting; June 3-7, 2022; Chicago, IL.