December 4, 2023
Once open-angle glaucoma is diagnosed and the need for therapy confirmed, treatment is started, and management commences. Intraocular pressure (IOP) becomes an important outcome measure in understanding if the condition is controlled, but there are a host of challenges as IOP is subject to measurement errors depending upon the tonometer used as well as the eye’s biomechanical properties such as corneal thickness and hysteresis.1,2 These issues are not new as the Ocular Hypertension Treatment Study brought attention to individuals with thin corneas having their IOP underestimated and those with thick corneas having higher measurements than what truly exist.3 Twenty years ago, people used pachymetry cards to correct IOP based upon corneal thickness, but it is now understood that it is impossible to correct the IOP.4
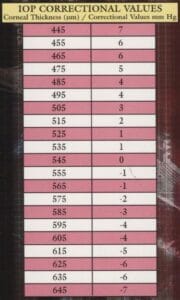
A thinner cornea will not only underestimate the IOP but is also associated with an increased risk of glaucoma developing or getting worse.5
IOP Usually Higher at Night
Robert Weinreb and his team at University of California at San Diego (UCSD) illustrated how IOP varies over a 24-hour period with IOP usually higher during nocturnal hours.6,7,8 This differed from conventional wisdom that had pressure readings highest in the early morning. The UCSD group used a sleep lab, pneumotonometer, and a technician who measured IOP while the person was awake and asleep (using night vision goggles to obtain the nighttime measurements). This led to a better understanding of how the IOP varies over a 24-hour period and began the quest for the development of tools that can measure IOP throughout the day and night.
Devices for Measuring and Monitoring IOP Readings
The Sensimed Triggerfish contact lens is an FDA-approved device capable of recording measurements over a 24-hour period.9
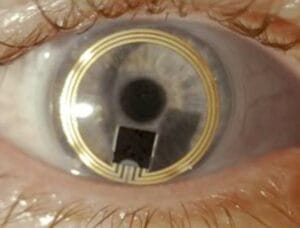
The readings are based upon corneal changes (strain gauge) and are not displayed in mmHg, which is problematic. The device never became commercially available in the United States but set the stage for future devices. The iCare Rebound tonometer (iCare, Inc.) is also FDA approved and was modified to create a home version that patients use to monitor their IOP readings.10

Implandata developed the Eyemate device that is implanted at the time of cataract surgery.11 The device, approved in Europe with a CE mark, has a microchip that records IOP over a 24-hour period. The patient holds a reader in front of the eye to download the readings using induction energy. The data is downloaded from the reader to the doctor’s computer when the patient returns for a visit. These 24-hour measuring devices confirmed the UCSD sleep lab studies results with the IOP usually higher during the nocturnal hours.11 Implandata is developing a device that sits in the suprachoroidal space, allowing the 24-hour monitoring device to be implanted in individuals with glaucoma who do not require cataract surgery.12
Day of the Week and Seasons Also Impact IOP
IOP varies throughout the day with pressure readings tending to be higher while individuals are sleeping.6,7,8 Due to the changing nature of IOP, clinicians should indicate to their patient that the tonometer reading just taken is a brief snapshot. What the pressure was an hour or month before is unknown, as is unknown the pressure when the person is sleeping or lying on their back.
Additionally, what about the IOP that is damaging to an individual’s eye is also unknown. IOP differs between eyes, and diurnal curves are not repeatable day to day.13,14 From 24-hour studies, we have learned that IOP tends to be highest on certain days and months, with measurements highest on Wednesday and lower on Fridays (the difference being just under one mm Hg).15 Also, the IOP varies between mid-winter and mid-summer months by 8.1% (2.5 mmHg), with the pressure being lower in the summer months.15.16 This work has been confirmed with variations in IOP also seen in normal-tension glaucoma.17
A paper by Mansouri and colleagues showed that the intraocular temperature varied day to day as well as seasonally, which may explain some of the variations in eye pressure.18 The authors note that there may be different reasons for the variations in ocular temperature besides the temperature outdoors such as lifestyle. A paper by Lee using the IRIS registry showed that IOP is higher in individuals who smoked or previously smoked cigarettes with the effect greater in those with glaucoma.19 The IOP difference was as much as 1 mm Hg. Alcohol usage is also associated with elevated IOP. 20,21
Using IOP as an outcome to determine if a person is controlled is challenging. Could the IOP be higher (or lower) due to the day of the week or month of the year the patient is being examined? Clinicians often use the concept of target IOP when deciding if a person is controlled, but there are reasons why the IOP varies that go beyond the patient not taking their medication. That is why, in addition to monitoring IOP, observing visual fields, optical coherence tomography scans, and optic nerve/retinal photos, watching for change is crucial to understand if your patient is controlled.
References
1 Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol 2000. 44 (5):367-408.
2 Sit AJ, Chen TC, Takusagawa HL, et al. Corneal hysteresis for the diagnosis of glaucoma and assessment of progression risk: A report by the American Academy of Ophthalmology. Ophthalmology 2023. 130 (4): 443-442.
3 Brandt JD, Beiser JA, Kass MA, et al. Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS). Ophthalmology 2020. 127 (4S):S72-S81.
4 Brandt JD, Gordon MO, Gao F, et al. Adjusting intraocular pressure for central corneal thickness does not improve prediction models for primary open-angle glaucoma. Ophthalmology 2012; 119 (3): 437-442.
5 Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors tht predict the onset of primary open-angle glaucoma. Arch Ophthalmol 2002; 120 (6): 714-720.
6 Liu JH, Kripke DF, Hoffman RE, et al. Nocturnal elevation of intraocular pressure in young adults. Invest Ophthalmol Vis Sci 1998; 39 (13):2707-12.
7 Liu JH, Kripke DF, Hoffman RE, et al. Twenty-four hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci 1999; 40 (12):2912-17.
8 Grippo TM, Liu JH, Zebardast N, et al. Twenty-four hour pattern of intraocular pressure in untreated patients with ocular hypertension. Invest Ophthalmol Vis Sci 2013; 54 (1):512-17.
9 Mansouri K, Weinreb RN, Liu JH. Efficacy of a contact lens sensor for monitoring 24-h intraocula pressure related patterns. PLoS One. 2015 (10) 5:30125530.
10 Takagi D, Sawada A, Yamamoto T. Evaluation of a new rebound self-tonometer, Icare Home: Comparison with Goldmann Applanation Tonometer. J Glaucoma 2017; 26 (7):613-618.
11 Mansouri K, Rao HL, Weinreb RN; ARGOS-02 Study Group. Short-term and long-term variability of intraocular pressure measured with an intraocular telemetry sensor in patients with glaucoma. Ophthalmology 2021; 128(2):227-233.
12 Szurman P, Gillmann K, Seuthe AM, et al. Eyemate-SC Study Group. Eyemate-SC Trial: Twelve-month safety, performance and accuracy of a suprachoroidal sensor for telemetric measurement of intraocular pressure. Ophthalmology 2023;130(3):304-312.
13 Realini T, Weinreb RN, Wisniewski SR. Diurnal intraocular pressure patterns are not repeatable in the short term in healthy individuals. Ophthalmology 2010. 117(9):1700-4.
14 Realini T, Weinreb RN, Wisniewski S. Short-term repeatability of diurnal intraocular pressure patterns in glaucoma individiuals. Ophthalmology 2011. 117(1):47-51.
15 Mansouri K, Gillmann K, Rao HL, et al. ARGOS-2 Study Group. Weekly and seasonal changes of intraocular pressure measured with an implanted intraocular telemetry sensor. Br J Ophthalmol 2021. 105 (3):387-391.
16 Morettin CE, Roberts DK, Newman TL, et al. Time-of-year variation in intraocular pressure. J Glaucoma 2021;30(11): 952-962,
17 Ikeda Y, Mori K, Ueno M, et al. Seasonal variation and trend of intraocular pressure decrease over a 20-year period in normal tension glaucoma patients. Am J Ophthalmol 2022;234:235-240.
18 Mansouri K, Gillmann K, Rao HL, et al. ARGOS-2 Study Group. Measurement of intraocular temperature in glaucoma: week-day and seasonal fluctuations. Br J Ophthalmol 2023;107:941-945.
19 Lee CS, Owen JP, Yanagihara RT, et al. Smoking is associated with higher intraocular pressure regardless of glaucoma. A retrospective study of 12.5 million patients using the Intelligent Research in Sigh (IRIS) Registry. Ophthalmology Glaucoma 2020;3(4):253-261.
20 Grant A, Roy-Gagnon MH, Bastasic J, et al. Alcohol consumption, genetic risk, and intraocular pressure and glaucoma: the Canadian Longitudinal Study on Aging. IOVS 2023;64 (10): 3.
21 Stuart KV, Luben RN, Warwick AN, et al. The association of alcohol consumption with glaucoma and related traits. Findings of the UK Biobank. Ophthalmology Glaucoma 2023;6:366-379.