December 20, 2023
As we wind down to the end 2023, the year will no doubt be remembered for finally having FDA-approved treatments for geographic atrophy (GA). That’s right, treatments plural; not just one but two. Pegcetacoplan (SYFOVRE) received FDA approval in February, followed five months later with the approval of avacincaptad pegol (ACP) (Izervay) in August. As exciting as that should be, it’s not been without some controversy. There are strong polarizing opinions regarding the overall efficacy of these drugs, some even questioning if the complement pathway is even an appropriate target for the treatment of GA. There are also concerns about the risk of near-blinding intraocular inflammation (IOI), which was seen with pegcetacoplan only after FDA approval.
For these reasons, GA continues to be one of the hottest topics in the retina community. At this year’s American Academy of Ophthalmology (AAO) meeting in San Francisco, three-year results from the GALE study (an extension of the DERBY and OAKS studies) and 24-month data from GATHER2 were presented, which will hopefully build some much-needed credibility for these two drugs.
OAKS and DERBY Studies
Pegcetacoplan was studied for the treatment of GA in the OAKS and DERBY studies, which were parallel Phase 3 studies comparing monthly (MO) and every other month (EOM) injections of pegcetacoplan vs. sham. The one-year results of the OAKS study showed statistically significant reductions in GA lesion growth vs. sham in the MO and EOM arms by 22% and 16%, but unfortunately DERBY did not reach statistical significance, showing only a 12% and 11% reduction in GA lesion growth in the MO and EOM arms, respectively.1 However, the 18-24-month results were much better. DERBY showed a 36% MO and 29% EOM reduction in GA progression, whereas OAKS had 24% MO and 25% EOM reduction in slowing GA progression.2 It was clear from the data that to see any kind of meaningful treatment benefit, patients would need to be treated at least through 18 months or longer to have a significant treatment benefit.
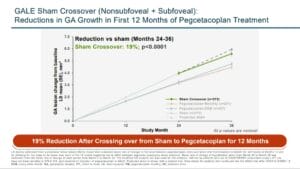
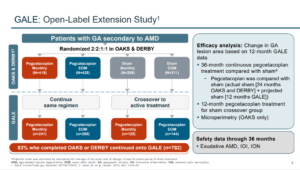
The GALE study was a three-year extension of the DERBY and OAKs studies consisting of 792 patients who were treated with pegcetacoplan MO or EOM out to three years. The trend of an accelerated treatment affect that was seen in the 18-24-month data continued out to three years where reductions in GA growth of 35% MO and 24% EOM was seen between months 24 and 36. Of particular interest, GA lesions that were non-subfoveal, showed a reduced growth rate up to 45% between treatment months 24 to 30 compared to the sham group.3
GATHER2 Study
Similar treatment results were also reported at the AAO with avacincaptad pegol (ACP) where investigators reported the two-year results from the GATHER2 study. GATHER2 was the second of two parallel studies comparing monthly injections of ACP with a sham injection in patients with GA. In GATHER2, after receiving monthly injections for the first 12 months, patients in year two were re-randomized to receive injections MO or EOM and followed out to 24 months.4 As was seen with pegcetacoplan, the longer duration of treatment resulted in a more significant reduction in the growth of GA. In GATHER2, the treatment effect with ACP more than doubled over two years compared to one year in both the MO and EOM versus the sham group. However, treatment MO compared to EOM did not result in a better job of slowing the growth rate of GA. Indeed, from 12 to 24 months, the EOM had a 19% reduction in growth rate of GA compared to a 14% reduction in the MO group.5
ACP’s Impact on Vision Loss Limited
What affect does treatment with ACP have on GA? Are we able to preserve or reduce the risk of vision loss with this medication? Interestingly, in post hoc analysis, investigators looked at the risk of > 15 letters of vision loss in the combined GATHER1 and GATHER2 groups compared to the sham group at 12 months and found that the treatment group showed a 56% risk reduction of persistent vision loss compared to the sham group. In other words, GA eyes that were not treated were more likely to progress and suffer persistent vision loss compared to the eyes that were treated. However, at 24 months with only the GATHER2 group, the vision benefit in treated eyes was no longer present.5 The mean change in visual acuity from baseline was similar between the treated and sham groups at two years. So, even though there was a doubling in reducing the growth rate of GA between 12 to 24 months, that did not necessarily reduce the risk of persistent vision loss as measured in the study. The risk of vision loss at 24 months between the two groups compared to baseline was the same.
Does that imply that by year two the disease continues to progress and ultimately as many people in the treatment group lost vision as the sham group? It seems counterintuitive if we are having a significant decline in the growth rate but ultimately not seeing any visual benefit. Perhaps this speaks to the nature of GA and/or how close these lesions were to the fovea at the time patients were randomized to treatment. This also underscores the absolute fact that neither of these treatments are a cure for GA but only hope to slow down disease progression.
Risks for IOI and CNV Evaluated
There have been concerns over the risk of IOI, particularly the risk of occlusive retinal vasculitis after injection of pegcetacoplan. This was reported by the American Society of Retinal Specialists in the summer of 2023. To date, there have been 10 post-approval confirmed cases of IOI with retinal vasculitis; three were occlusive and three were non-occlusive, with all occurring after the first pegcetacoplan injection. With over 100,000 injections, the rate of retinal vasculitis events is estimated to be around 0.01% per injection. Interestingly, there have not been any cases of occlusive retinal vasculitis in the registration trials with either pegcetacoplan or ACP. Nor has there been any post market reported cases of occlusive retinal vasculitis with ACP.
Another issue that has been raised (with both medications) is the incidents of choroidal neovascularization (CNV), which seems to occur more frequently in eyes that are treated compared to the sham group. In DERBY and OAKS with pegcetacoplan at 24 months, MO and EOM arms had a slightly higher rate of CNV (7.4%) compared with 4% – 5% with the sham group. In the first year of the GALE extension study (in which there was not a sham group) the rate of new-onset exudative AMD was 7.1% (MO) and 2.3% (EOM).
The incidence of CNV with ACP is also slightly higher in treated eyes. After the first year, the incidence of CNV in the treated eyes was 6.7% vs. 4% in the sham group; the rates were similar at year two with 7% developing CNV vs. 4% in the sham. In total over two years 11.6% developed CNV vs. 9% in the sham group.
There is clearly a higher risk of CNV with both drugs, but it’s not like the sham eyes were immune to developing CNV. Indeed, they did develop CNV just not at quite the same frequency. This underscores the progressive nature of macular degeneration and the fact that even if we are targeting the advance dry form of the disease, there is still a risk of conversion to the wet form of AMD. Likewise, even in the anti-VEGF studies for wet AMD, patients that were well controlled with an anti-VEGF medication often lost vision, because of GA.
High Hopes for Future GA Treatments
This year has been the year of GA, at least in the retina world. We have two treatments that have clearly and indisputably demonstrated efficacy in slowing the progression of GA, and the data shows that these medications work even better the longer patients are receiving treatments. At this point we don’t know which drug is better as the clinical trial designs were different between the two medications, and they have not been compared in a head-to-head trial.
As we look forward to 2024, we will learn even more as these medications become more widely adopted. Many of the questions that we had in 2023 will become even better elucidated as more and more of our patients get treated. Though we aren’t curing GA, we are slowing it down with the hope that it’ll be enough for each patient who sits in our exam chair to live an active and productive life that is not limited by their visual function. That is the hope for 2024, that we do it better, recognize GA earlier, refer when appropriate, and offer hope to our patients that didn’t exist when we started 2023.
References
1 Apellis. Apellis announces pegcetacoplan showed continuous and clinically meaningful effects at month 18 in phase 3 DERBY and OAKS studies for geographic atrophy (GA). Accessed 12/4/2023
3 GALE Extension Study Shows Syfovre Continued to Demonstrate Increasing Treatment Effects Over 3 Years in Patients with GA. Eyewire. 11/4/2023; Accessed 12/4/23.
4 Khanani AM, Patel SS, Staurenghi G. et al. The Efficacy of Avacincaptad Pegol in Geographic Atrophy: GATHER1 and GATHER2 Results. Retina Society 2022. Accessed 12/4/2023
5 Charters Lynda. AAO 2023: Iveric Bio announces positive Phase 3 results for avacincaptad pegol intravitreal solution for GA secondary to AMD. Ophthalmology Times. 11/4/23