June 26, 2023
In the current era of modern technology, why are we still recommending an Amsler grid to our patients with macular degeneration? The Amsler grid dates back to 1947 and is named after Swiss ophthalmologist Marc Amsler. The genesis of the grid may go even further back as Amsler himself referred to a paper from 1894 that consisted of parallel lines to determine the presence of “metamorphoma.”1
The Amsler grid is ubiquitous to every optometrist and ophthalmologist office in the world. Don’t get me wrong, it was probably considered groundbreaking at the time, but wouldn’t you think after 76 years we could develop something better and more sensitive to detect macular abnormalities than an Amsler grid?
If you have ever watched a patient perform an Amsler grid, you can often see their eyes darting everywhere, and you can’t help but wonder how good the grid is at detecting areas of distortion. The fact is, not very. In one study, the sensitivity was shown to be only 56% and others as low as 34%.1 In a recent meta-analysis on the diagnostic accuracy in detecting nAMD, the authors found that the Amsler grid sensitivity was low and concluded the sensitivity might be at levels typically not recommended for monitoring.2
Fortunately, we have better technologies. It’s just a matter of changing the traditional dogma and recognizing that as eye care providers we need to do better for our patients. There is too much at stake given the era we are in of effective treatments for nAMD. The fact is, today’s modern technologies are way, way more sensitive than the Amsler grid at detecting early change, and as a result we have the opportunity to do better for our patients.
The New Era of AMD Treatments
There has been a lot of attention on macular degeneration the past few years with the approval of several “next generation” treatments. As good as the new treatments hope to be, often the limiting factor in determining a good visual outcome is not which medications gets used to treat nAMD, but rather the level of acuity the patient has when nAMD is diagnosed. Indeed, baseline visual acuity has been shown to be the strongest predictor of what the visual outcome will be after one year.
In the Complications of Age-related Macular Degeneration Treatment Trial (CATT) comparing ranibizumab to bevacizumab, patients in both groups demonstrated a significant improvement in final visual acuity, but patients who started with a VA of 20/40 or better were more likely to maintain that level of acuity at one year compared to patients whose visual acuity was 20/100 or worse at the time of diagnosis. Patients with worse VA (at the time of diagnosis) did show improvement, but most were never able to catch up. Sadly, only 36% of patients in the CATT trial were 20/40 or better at the time of diagnosis.3 Unfortunately, outcomes such as this are all too common. In a recent study from the IRIS registry consisting of almost 160,000 eyes treated for nAMD, the average VA at the time of diagnosis was around 20/80, and only 34% of patients were 20/40 or better at the time of diagnosis.4
Apps Are Faster Way to Monitor for AMD
So how can we do a better job of recognizing choroidal neovascularization (CNV) earlier, before patients lose significant visual acuity? It’s certainly not going to be with using an Amsler grid! There are two smartphone-based apps that have been FDA cleared to monitor AMD, Alleye and My Vision Track (mVT). Alleye implements an alignment hyperacuity task that allows early detection of significant changes in visual function. The test requires patients to align a mobile central dot along two fixed flanking dots to an imaginary straight line. This task is repeated three times in differing positions with different distances between central and flanking dots in horizontal, vertical, and oblique axes. Unlike the Amsler, the Alleye assures patients fixation on the mobile central dot.5 It has been validated in several clinical trials.6
In addition to monitoring macular function, the Alleye app also allows patients to enter and view their diagnosis and keep track of doctor appointments as well as medical results, such as central retinal thickness and visual acuity. Patients also can monitor their Patient Reported Outcome Measures (PROMS) as well as data of the last injection.
The My Vision Track (mVT) 7 app was the first FDA-cleared vision-monitoring app designed to detect early vision changes in patients with AMD and diabetic eye disease. It is a “shape discrimination” test that uses fully or partially modulated or unmodulated circular Gaussian patters as stimulus. Each modulated pattern is displayed at a random position, and users are asked to differentiate between them while holding the device at around 30 cm. Patients perform the test twice a week, and the results are automatically sent to the physician. If there are any abnormalities or changes in the test, an alert is sent out.
The app is free, but unfortunately it’s available to patients only by a prescription from a retinal specialist. The company has a partnership agreement with Genentech, though it is not clear if this app is still supported by Genentech.
ForeseeHome Detects Early Distortion
Perhaps the most widely adopted technology for monitoring AMD is ForeseeHome by Notal, which uses preferential hyperacuity perimetry as a way of detecting early distortion from macular degeneration. It is a computer-based home device that is performed by the patient. The test creates an artificial distortion that the patient is able to map out. If a patient converts to CNV, the fluid and irregularity that develop in the retinal architecture will create more distortion than the baseline test. When this occurs, an alert gets sent to a reading center, and the data is analyzed by artificial intelligence (AI) software for aberrations in the test results. If the alert is confirmed, an in-house ophthalmologist at the Notal Vision Monitoring Center reviews the results and determines if an in-person exam is recommended.8 The patient’s provider is then contacted so the patient can be seen with the hope of earlier diagnosis and treatment.
In order for patients to have the ForeseeHome device prescribed, they must have intermediate level AMD and have a visual acuity of 20/60 or better. There is a monthly fee, but traditional Medicare and other commercial insurances typically cover 80% of the cost; supplemental insurances will cover the remainder. For those without supplemental insurances, the national average monthly cost is around $15.00
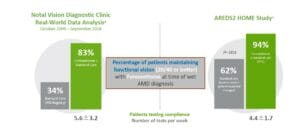
The ForeseeHome device seems to be very sensitive at detecting early conversion from dry to wet AMD. One arm of the AREDS2 evaluated patients followed with the ForeseeHome device and showed 94% of patients who converted from dry to wet presented with visual acuity of 20/60 or better when using the ForeseeHome device compared with only 62% who relied on routine visits and patient-reported symptoms.9 In another study using a large dataset from the IRIS registry, 83% of patients presented with 20/40 or better VA compared to only 34% who were not using the ForeseeHome device. This “real-world” study from the IRIS registry clearly shows the benefits of computer-based home monitoring compared to the usual care.10
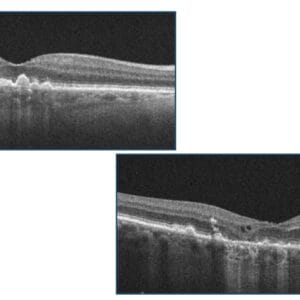
Finally, why is early detection of dry to wet AMD so important? In the ALOFT study (Analysis of Long-term Visual Outcomes of ForeseeHome Remote Telemonitoring) of 2,123 dry AMD cases followed over 10 years, patients whose conversion to wet AMD detected by ForeseeHome had an average VA of 20/32 at 2.7 years of treatment compared to 20/80 after two years with current standard of care monitoring.11
The impact of avoidable vision loss on our patients in the era of new treatments and new technologies should inspire us to do more than just recommend a piece of paper with a checkered grid that dates back to the 1940s. Instead, we need to make sure our patients have the best opportunity to detect problems sooner, so they can be diagnosed and treated earlier. The evidence shows that when this happens, patients have better visual outcomes.
Certainly, the economics of a computer-based home monitoring system must be considered; but the costs do not seem unreasonable in the big picture of health care expenditure costs, especially when you consider the impact on the lives of our patients who suffer significant vision loss. Although the Amsler grid is free and is easily understood by our patients, the fact is, it does a poor job of helping to monitor patients with dry AMD. We can do better…we have to do better for our patients!
References
1 Crossland M, Rubin G. Amsler chart: absence of evidence is not evidence of absence. Br J Ophthalmol 2007 Mar; 91(3): 391–393.
2 Bjerager J, Schneider M, Potapenko I, et al. Diagnostic Accuracy of the Amsler Grid Test for Detecting Neovascular Age-Related Macular Degeneration: A Systematic Review and Meta-analysis JAMA Ophthalmol. 2023;141(4):315-323. doi:10.1001/jamaophthalmol.2022.6396.
3 Ying GS, Huang J, Maguire MG, et al. Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013; 120(1):122–129. 10.1016/j.ophtha.2012.07.042PMID:23047002
4 Ho AC, Kleinman DM, Lum FC, et al. Baseline Visual Acuity at Wet AMD Diagnosis Predicts Long-Term Vision Outcomes: An Analysis of the IRIS Registry, Ophthalmic Surgery, Lasers and Imaging Retina, 2020;51(11):633–639
5 Alleye accessed June 16, 2023
6 Schmid, M.K., Thiel, M.A., Lienhard, K. et al. Reliability and diagnostic performance of a novel mobile app for hyperacuity self-monitoring in patients with age-related macular degeneration. Eye 33, 1584–1589 (2019).
7 MyVision Track accessed June 16, 2023
8 Busquets MA. Artificial Intelligence and Augmented Intelligence in Ophthalmic Practice: New technologies enhance patient experiences and maximize the ability of ophthalmologists to practice effectively. Retinal Physician. Jan 2023
9 Chew EY, Clemons TE, Bressler SB, et al; AREDS2-HOME Study Research Group. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology. 2014;121(2):535-544.
10 Real-World Performance of a Self-Operated Home Monitoring System for Early Detection of Neovascular AMD (ForeseeHome device), presented by Allen Ho, American Society of Retina Specialists Meeting 2020.
11 Mathai M, Reddy S, Elman MJ, et al. Analysis of the Long-term Visual Outcomes of ForeseeHome Remote Telemonitoring: The ALOFT Study. Ophthalmology Retina. 2022;6 (10): 922-929