April 24, 2023
There has been a lot of attention around age-related macular degeneration (AMD) over the past several months with the approval of pegcetacoplan for the treatment of geographic atrophy (GA) as well as newer second generation treatments for neovascular AMD (nAMD).
Indeed, with AMD being recognized as the leading cause of legal blindness over the age of 65, the attention on AMD is well deserved. When you consider we have an aging population with people living longer and more productive lives, there will be no shortage of AMD patients coming into our offices.
Fortunately, a significant percentage of OD practices now have OCTs, so as primary eye care providers we should be well equipped to monitor these patients for conversion to either nAMD or recognize the presence of early GA.
Identifying Higher Risk Patients
With every dry AMD patient, we categorize or stage the level of AMD as a means of gauging the risk for progression to nAMD or GA, but the risk can vary widely depending on the level of AMD. Indeed, AREDS determined that the five-year risk for progression from early and intermediate AMD to advanced disease varied from 0.04% to 53% with intermediate AMD patients having a higher risk for conversion.1 The staging for AMD is done largely based on our clinical exam. Can we combine what we see in our clinical exam with OCT findings and do an even better job of identifying those higher risk patients?
The focus for most of us when looking at a B-scan of an AMD patient is determining if there is conversion from dry AMD to wet. We look carefully for the presence of subretinal or intraretinal fluid. We look for elevation of the retinal pigment epithelium (RPE) that may be indicative of a CNV or a higher reflective area that might represent the presence of a CNV. With the approval of pegcetacoplan we are now looking more carefully for GA on the B-scan’s and the enface images.
OCT Biomarkers May Predict nAMD or GA
Evaluating an OCT scan for these changes is fundamental in the management of AMD, but if we look more carefully there may be other features on the OCT that may help us do an even better job of identifying higher risk patients. Indeed, researchers have identified certain biomarkers on an OCT scan, that when present, may be predictive for progression to nAMD or GA. These biomarkers include:
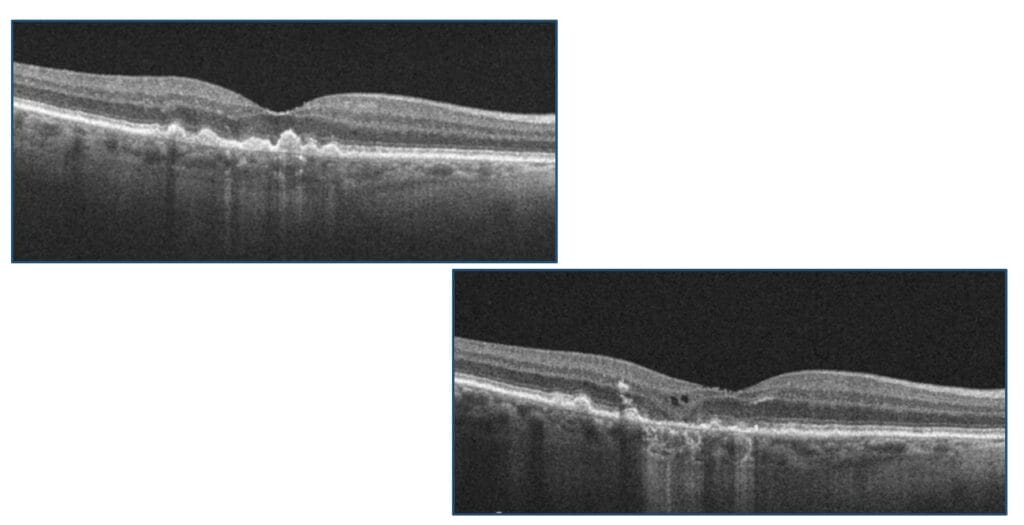
- Drusen volume and increased drusen height
Drusen has long been associated with the development of advanced or late stage AMD. It stands to reason that the more drusen there are, the greater the risk for progression or conversion. In addition, increased drusen height and drusen length have also been associated with an increased risk of conversion to nAMD. This is visualized on funduscopy and can be correlated with the OCT findings. OCT device companies have software that can calculate drusen volume in addition to progression (or regression) of drusen over time.
Figure 1
Drusen regression is also an important risk factor for progression to GA. According to AREDS results, 82% of eyes that showed drusen regression developed significant atrophic RPE and hypopigmentary changes.2
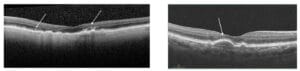
Figure 2
These are seen as bright hyperreflective spots above the RPE in the sensory retina. They represent extracellular pigment granules and outer segment debris (outer HRF). They may also represent displacement and clumping of degenerated RPE cells
In AREDS2, patients with HRF had a five-time increased risk of progression to GA at two years vs. controls.3
Figure 3
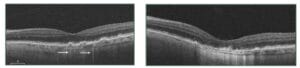
- Subretinal drusenoind deposits (SDD), also known as reticular pseudo drusen
SDD represent subretinal car, interlacing, hyperreflective material located above RPE. They are often seen superior to the macula or close to superotemporal arcade. They can undergo a characteristic lifecycle of growth, invasion into the ellipsoid zone, and finally regression. Reticular pseudodrusen is associated with an additional two- to six-fold increased risk of progression to nAMD or central GA.4-5
Figure 4
- Hyper-transmission defects
These represent narrow strips of light transmission into the choroid in the presence of what appears to be an overlying intact RPE. They may represent micro-cracks within the RPE. Nonetheless, there is an increased risk of progression to GA or nAMD when they present. In one study, when hyper-transmission defects were present, 27% of eyes progressed to GA or nAMD.6
Figure 5
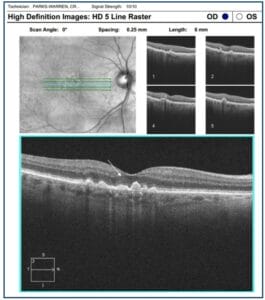
- Incomplete Retinal Pigment Epithelial and Outer Retinal Atrophy (iRORA)
The term iRORA refers to localized loss of tissue in the outer retinal layers without definite RPE loss and is strongly correlated with the progression to GA. iRORA is a new term defined by the Consensus International Group (CAM—Classification of Atrophy Meeting) and has replaced the previous term called “Nascent GA.” iRORA is a precursor to cRORA, which represents complete loss of the RPE and outer retinal layers and is indicative of GA. In one study the conversion to GA in patients with iRORA occurred on average by 11 months.7
Figure 6
The risk for progression in dry AMD to advanced disease can vary widely, and though clinical findings can be helpful in identifying those who are at higher risk, recognizing critical biomarkers on OCT may help us do an even better job. Ultimately, this would lead to earlier recognition when patients convert to neovascular disease and/or GA, which in turn would lead to earlier treatments and better visual outcomes.
When you consider the important role optometry plays as part of the eye care team in diagnosing and managing patients with AMD, it’s important that we take OCT interpretation to the next level. The recognition of important biomarkers in AMD is part of the continuum of OCT interpreting in AMD.
A few years ago, I heard Brad Sutton, OD, a well-known optometrist at Indiana University School of Optometry in Indianapolis, talk about these biomarkers in a lecture, and he made the comment, “Once you see them, you can’t unsee them.” Brad was so right! These biomarkers are unique and are easily seen in dry AMD if only you look for them.
References
1 Ferris, F.L., Davis, M.D.; Clemons, T.E.; et al. Age-related eye disease study research group. A simplified severity scale for age-related macular degeneration: AREDS ReportNo. 18. Ophthalmol 2005, 123, 1570–1574.
2 Klein, M.L.; Francis, P.J.; Ferris, F.L., 3rd; et al. Risk assessment model for development of advanced age-related macular degeneration. Ophthalmol. 2011, 129, 1543-1550.
3 Christenbury, J.G.; Folgar, F.A.; O’Connell, R.V.; et al. Age-related eye disease study 2 ancillary spectral domain optical coherence tomography study group. Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology 2013, 120, 1038–1045.
4 Zweifel, S.A.; Imamura, Y.; Spaide, T.C.; et al. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology 2010, 117, 1775–1781.
5 Flores, R., Carneiro, Â., Tenreiro, S., et al. Retinal Progression Biomarkers of Early and Intermediate Age-Related Macular Degeneration. Life 2022, 12, 36.
6 Padnick-Silver L, Weinberg AB, Lafranco, F.P., et al. Pilot study for the detection of early exudative age-related macular degeneration with optical coherence tomography. Retina 2012, 32, 1045–1056.
7 Wu, Z., Luu, C.D., Ayton, L.N., et al. Optical coherence tomography-defined changes preceding the development of drusen-associated atrophy in age-related macular degeneration. Ophthalmology 2014, 121, 2415–2422.