January 25, 2024
Interventional glaucoma is a concept commonly discussed at glaucoma meetings with the term meaning different things to different people. To some it implies that glaucoma is best managed surgically with the term intervention signifying a surgical procedure. I, however, think of interventional glaucoma in broader terms, implying that therapeutic interventions are personalized based upon patient need and include all modalities (medication, drug delivery, laser, surgical).
The glaucoma treatment algorithm not long ago went from medical therapy to laser trabeculoplasty (therapy was advanced if the intraocular pressures were too high or progression noted) with filtration surgery the final step. With the introduction of new therapeutic modalities and procedures such as the minimally invasive glaucoma surgeries (MIGS), laser trabeculoplasties, drug-delivery devices, and new medications, the clinician is offered many options. Interventional glaucoma implies a mindset in which therapy is proactive, done in a timely fashion, recognizing the relentless nature of glaucoma. The patient’s age, race, ocular history, medical history, and family history, as well as patient and doctor anxiety are considered. This approach differs from traditional management in which the patient must get worse such as when progression is demonstrated before therapy is advanced.
iDose and Durysta Added to Glaucoma Care Options
Over the past decade, there have been many therapeutic advances. On the medical side, the rho kinase inhibitors (ROCK) and nitric oxide molecules have been introduced. These advances allow significant intraocular pressure (IOP) reduction and perhaps enhanced blood flow from the use of medication(s). Two drug-delivery devices have been approved, iDose (Glaukos) and Durysta (AbbVie), which allow medication to be placed into the anterior chamber with a prolonged effect, improving adherence.

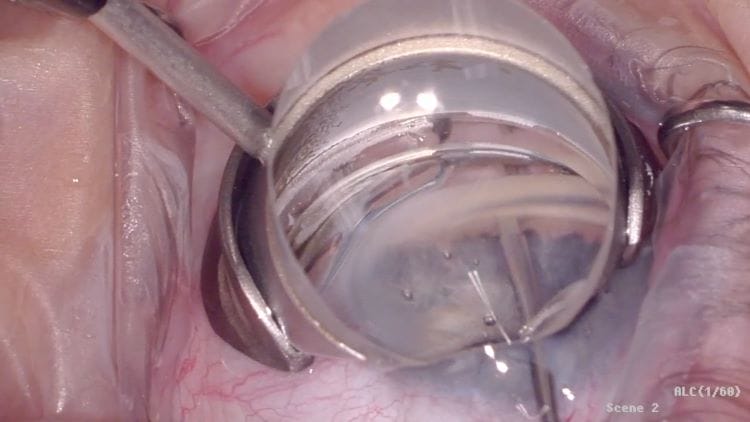
The direct selective laser trabeculoplasty (DSLT) device (Belkin Vision) was recently FDA approved, which will improve the procedure’s efficiency. DSLT allows laser trabeculoplasty to be performed without touching the eye or the need for gonioscopy, as the platform places laser marks onto the trabecular meshwork (TM) from outside the eye. A huge advance has been in the types of MIGS procedures available. The iStent was introduced more than a decade ago, approved for use at the time of cataract surgery. The iStent is a small tube-like device that is inserted into the trabecular meshwork, allowing aqueous to flow from the anterior chamber into Schlemm’s canal. Since its introduction, other iStent devices have been introduced, including the iStent inject W (two stents placed a few clock-hours apart) and the iStent Infinite (three stents, which can be inserted in a freestanding procedure)

The Hydrus Microstent (Alcon) is a tiny scaffold inserted into Schlemm’s canal, which also allows aqueous to bypass the TM in which a single stent covers three clock-hours. This also needs to be inserted in conjunction with cataract surgery.
Goniotomy and Canaloplasty
The next category of MIGS is included among Ab-interno procedures, such as goniotomy and canaloplasty, in which a small catheter is inserted into Schlemm’s canal. I think of these as a plumber cleaning out a pipe, and a viscoelastic is often placed into the TM to dilate the canal. One version is ABiC (Ab-interno Canaloplasty), which flushes out the outflow channels, improving aqueous drainage.
Another type of surgery is goniotomy, in which aqueous flow is enhanced by removing the juxtacanalicular tissue and inner wall of Schlemm’s canal, allowing better access into the TM. The KDB Glide (New World Medical), which is the newest version of a device originally called the Kahook Dual Blade, uses blades to remove TM tissue.
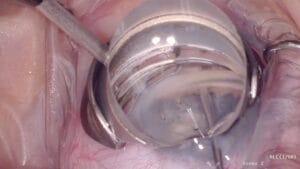
A version of goniotomy is with the use of a Trabectome, which is an electrosurgical device that excises the TM. Most MIGS procedures do not reduce IOP to the extent seen with a trabeculectomy, but complications are reduced. Most MIGS procedures are indicated for mild to moderate stages of glaucoma. The Xen Gel Stent (Allergan, AbbVie), also labeled as a MIGS procedure though it is more invasive, uses a small stent running from the anterior chamber into the subconjunctival space, and it allows greater IOP reduction, leading to its indication for more severe forms of glaucoma.
Procedure Cost and Reimbursement Concerns
MIGS surgeries have improved glaucoma care, allowing therapy to be individualized. A greater number of MIGS procedures are being performed, as compared to more traditional surgery.1 A clinician can select a treatment plan, ranging from medications to SLT to drug-delivery devices to MIGS devices to filtration surgery, which is huge advance from just a few years ago. By using MIGS procedures, glaucoma operations once only performed by glaucoma surgeons are now often performed at the time of cataract surgery by general ophthalmologists.1 The improvement in care has come with increased costs. Lee discussed the increase in Medicare part B payments for selected glaucoma procedures, which increased from $52.0 million in 2007 to $179.9 million in 2017.2
Probably due to the increasing costs, several Medicare administrative contractors (MAC) released drafts of local coverage determinations (LCD) in the summer of 2023 that illustrated potential reimbursement in 2024. Changes discussed were going to reduce or eliminate reimbursement for a host of MIGS procedures including goniotomy, canaloplasty, and even a non-MIGS procedure, cyclophotocoagulation. Not included in the reimbursement cuts were iStent and Hydrus procedures. The MAC’s rationale was that these procedures were experimental or not supported by research. But these procedures were not new, had a host of research behind them, and had become part of the glaucoma treatment regimen by many clinicians. The uproar was intense as the major ophthalmological associations provided feedback, explaining that the drafts were in error and not conducive to improving patient care. In December, the MACs announced that the changes would be tabled and not implemented in 2024. But they did not say that they were eliminating the chance for future reimbursement cuts. One would think that if a procedure undergoes a successful FDA review (which is robust, onerous, lengthy, and difficult), this should be sufficient to ensure reimbursement. Still, with the reality of limited budgets (nobody wants to see their taxes go up), tough decisions may still occur, but the process of what is covered by Medicare could be smoother.
Glaucoma care is evolving, with patients having greater therapeutic options so that care can be personalized. It is concerning that costs may interfere with how a doctor may take care of their patients. It’s an ongoing issue. Still, this is a great time for glaucoma care with a host of new glaucoma modalities available.
References
1 Rathi S, Andrews CA, Greenfield DS, et al. Trends in Glaucoma Surgeries Performed by Glaucoma Subspecialists versus Nonsubspecialists on Medicare Beneficiaries from 2008 through 2016. Ophthalmology 2021; 128(1):30-38.
2 Lee JH, Anthony KM, Warren JL, et al. Impact of iStent Microbypass Shunt on Medicare Part B Glaucoma Surgical Expenditure. Ophthalmol Glaucoma 2021; 4(2): 131-138.