December 7, 2023
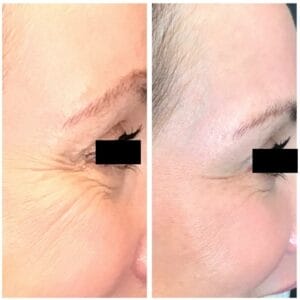
What a fun year this has been thus far learning alongside you about aesthetics in eye care. As we move toward the end of the year, it’s a good time to summarize and plan those holiday parties for your office. Every year our office hosts our annual “Bubbles & Botox” event in December. No, I am not condoning serving alcohol and then performing injections, but I am suggesting you take this opportunity to show your patients everything you have to offer. Our event consists of stations where our invited patients can move through and learn about IPL, radiofrequency, Upneeq/Vuity, skin care and ingredients, sunglass and ophthalmic frame styles, and, of course, get a consult for neurotoxin injections. We have drawings, swag bags, and holiday giveaways. At each station they get a stamp on a bingo card that puts them closer to another giveaway. This has become an event that patients love and ask about in advance to ensure they get their invite. We typically have about 80 people come and go, it’s like a trunk show but on steroids. See Figures 1 and 2.


Start by Evaluating the Periocular Area
If you are hosting these types of events, it’s really important to be able to properly evaluate patients’ needs. Assessing the periocular area is paramount to understanding what solutions are available and incorporating those into our practices. Here’s how to assess the periocular area.
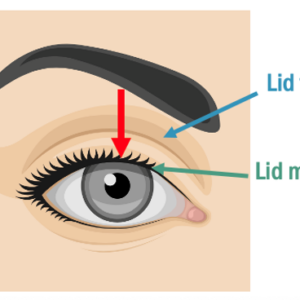
Start by looking at the entire face and how everything works together. The periocular area begins at the top of the forehead and continues through the lower edge of the midface as shown in Figure 3.
Look at where the brows sit and if there is frontalis recruitment to pull up the brows or help alleviate dermatochalasis. Observe any chin lift due to heavy lids. Look at eyebrow and eyelids for lid plate and marginal reflex distance-1 (MRD-1), as shown in Figure 4.

Where do the lower eyelids sit in conjunction to the globe? Finally assess the midface and malar region. Look at the skin for signs of rosacea-telangiectasia’s or ruddiness. Is there volume loss in the midface that is creating tension on the lower eyelids resulting in ectropian or poor eyelid seal as shown in Figure 5?

Once you’ve made your assessment you can begin to incorporate your energy-based devices, therapeutics, and neurotoxin. IPL works beautifully on managing rosacea and photodamage. RF’s tightening effects help to rebuild collagen, restore volume loss, as well as increase blood flow. Lasers can do all of the above. Neurotoxin is a fierce weapon in rhytid formation because it temporarily stops the muscle movement that creates wrinkles. Minor surgical procedures can clean up lumps and bumps that patients may find unsatisfying. We have quite an armamentarium of therapeutic drops between Upneeq, Lumify, XDemvy, and all our dry eye medications that help patients regain their sparkle due to their healthier tear film.
Modernization of Cosmetics Regulation Act
Together, we have extensively covered ingredients and how our patients should care for their eyes on a daily basis. One new resource we have and something encouraging is the Modernization of Cosmetics Regulation Act of 2022 (MoCRA). This was signed into law on Dec. 29, 2022, and goes into effect on Dec. 29, 2023, barring any changes that occur prior to this date. This is the first time the FDA has made a significant change to the regulation of cosmetics since 1938. This law should provide a major overhaul to the existing cosmetic regulations. Part of this overhaul is to create “Good Manufacturing Practices” for all companies that manufacture cosmetics. The law requires reporting of any serious adverse health events that are caused by a cosmetic. This would be considered mandatory. As I read it, my hope would be that it would have a similar requirement like that of FAERS, the FDA Adverse Event Report System. This public dashboard is a web-based interactive tool that the public utilizes, including eye care professionals, to report adverse events in relation to FDA-approved medications. Also listed in MoCRA is mandatory testing of asbestos levels. Cosmetic listing requirements have been quite lax depending on the amount of the ingredient. This new law will also include updates to cosmetic listing requirements through an online portal system but not through structured product labeling. As this comes into effect, there will be many more learnings.
As we all learn together, I encourage you to incorporate these new observations into your practice, find ways to confidently have conversations with your patients, and grow your practice by adding value to your current patient base. Add technology as you build your protocols and thrive in implementing these new skills. Glow on!